스테로이드 치료 후 조직학적으로 확인된 C1q Nephropathy 완전관해
Complete Remission from C1q Nephropathy with Disappearance of C1q Deposition after Steroid Therapy
Article information
Trans Abstract
C1q nephropathy is a rare glomerular disease, defined by characteristic mesangial C1q immune deposition seen in immunofluorescence microscopy with no serological evidence of systemic lupus erythematosus. C1q nephropathy can be diagnosed with a subsequent biopsy, as with IgA nephropathy. There are some cases with an initial diagnosis of hematuria and proteinuria with minimal disease changes, focal segmental glomerulonephritis, and mesangial proliferative glomerulonephritis, but lacking C1q nephropathy, in which C1q deposition on immunofluorescence subsequently develops. We report a case that was diagnosed as diffuse mesangial proliferative glomerulonephritis, but a subsequent biopsy showed C1q nephropathy, with C1q deposition in both immunohistochemistry and electron microscopy (EM). We treated the C1q nephropathy with methylprednisolone and confirmed the disappearance of C1q depositions by both immunohistochemistry and EM in a follow-up biopsy.
INTRODUCTION
C1q nephropathy is a rare immunohistopathological disease, first described by Jennette and Hipp [1,2]. The diagnostic criteria for C1q nephropathy are the characteristic deposition of C1q in the renal mesangium on immunofluorescence with no clinical or serological evidence of systemic lupus erythematosus (SLE) [1,2]. The prevalence of C1q nephropathy in renal biopsies is from 0.2% to 16%, and it predominantly affects children, males, and African-Americans [3,4]. The clinical features are diverse, ranging from asymptomatic hematuria or proteinuria to nephritic syndrome, with or without hypertension and renal insufficiency [3,4]. Light microscopic features are heterogeneous and vary from minimal disease changes, focal segmental glomerulosclerosis, and proliferative glomerulonephritis [3-5].
C1q nephropathy is usually considered steroid-resistant. The response to immunosuppressive therapy is worse, especially when associated with nephrotic syndrome or with focal segmental glomerulosclerosis (FSGS) [5,6].
In this report, we describe the case of a 5-year-old female who presented with persistent microscopic hematuria and was diagnosed with C1q nephropathy after a renal biopsy. We treated her with three cycles of methylprednisolone pulse therapy. After this, she showed complete remission, with no proteinuria or hematuria for 6 months. A follow-up renal biopsy showed the complete disappearance of C1q deposition by immunofluorescence microscopy and electron microscopy (EM) showed no electron-dense deposit.
C1q nephropathy is rare and can be misdiagnosed at a first biopsy because of the subsequent development of C1q deposition. To our knowledge, this is the first reported case in South Korea showing complete remission with steroid therapy, with the confirmed absence of C1q deposition.
CASE REPORT
A 5-year-old female presented with intermittent hematuria. She had been diagnosed with diffuse proliferative mesangial nephritis but showed persistent hematuria with an angiotensin II receptor blocker at a university hospital. She visited our clinic because of persistent hematuria and urinary frequency. Urinalysis showed white blood cell (WBC) 1+ and red blood cell (RBC) 2+. She was diagnosed with cystitis and treated with antibiotics. Despite this treatment, she presented with intermittent hematuria twice more. Thus, we performed a renal biopsy again before considering immunosuppressive treatment.
Laboratory findings before the biopsy were as follows: hemoglobin 12.4 g/dL, hematocrit 39%, white blood cells 5,800/mm3, platelets 239,000/mm3, serum total protein 6.5 g/dL, albumin 4 g/dL, cholesterol 143 mg/dL, triglycerides 66 mg/dL, aspartate aminotransferase 31 U/L, alanine aminotransferase 16 U/L, blood urea nitrogen (BUN) 15.8 mg/dL, creatinine 0.56 mg/dL, C3 106 mg/dL, C4 12 mg/dL, HBsAg (−), and anti-HBs (+). Urinalysis showed protein (−), glucose (−), RBC (−), and WBC (−), spot urine protein-to-creatinine ratio 0.065, and estimated glomerular filtration rate (eGFR) 194 mL/min. Her antistreptolysin O titer was not elevated. Anti-nuclear antibody and anti-DNA antibody tests were negative.
A percutaneous renal biopsy was performed under local lidocaine anesthesia. The biopsy needle (TSK Acecut biopsy needle) was inserted at the lower pole of the kidney under ultrasound guidance (GE Logi Q9). Biopsy samples were sent to Korea Pathology Lab immediately after the biopsy.
The specimen for light microscopy contained mostly renal medulla and only one glomerulus. The specimen for electron microscopy contained two glomeruli. We show a photomicrograph of a semi-thin section stained with toluidine blue (Fig. 1A and 1B). Light microscopic findings showed that tubules had slight focal atrophy and loss with interstitial fibrosis (Fig. 1A and 1B). Tissue sections were perfused with 2.5% glutaraldehyde. After post-fixation in 2% osmium tetroxide, they were dehydrated and embedded in Epoxy resin. Ultrathin sections were stained with 7% uranyl acetate and 1% lead citrate, and observed with a H-7650 transmission electron microscope (Hitachi, Japan). The electron microscopy specimen showed small amounts of mesangial deposits. The glomerular basement membrane was normal in thickness, with partly irregular inner contours. Epithelial cell foot processes showed slight focal effacement. On EM, there were dense deposits in the widened mesangial matrix beneath the glomerular basement membrane (Fig. 1C and 1D). Immunofluorescence microscopic findings were consistent with immune-mediated nephropathy and were suggestive of C1q nephropathy, with dominant mesangial C1q staining (Fig. 1E and 1F).
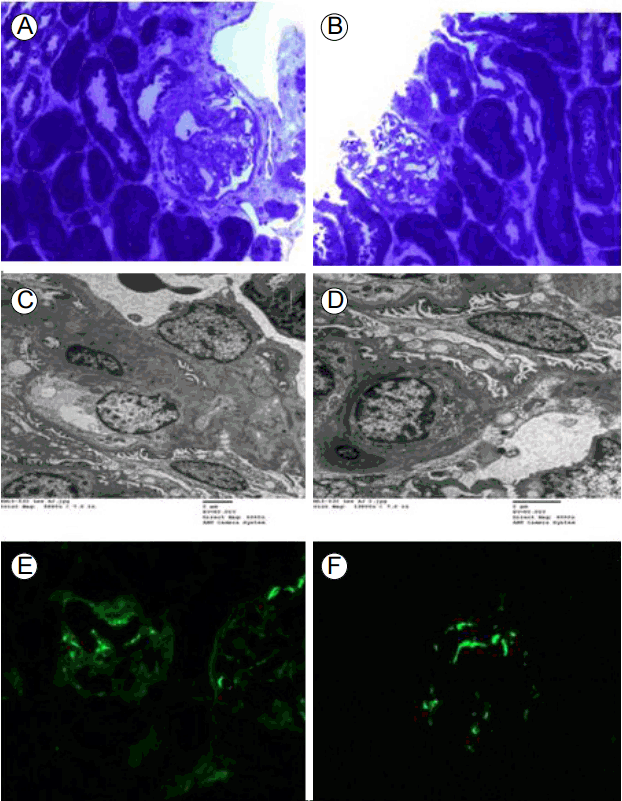
Pathological findings in C1q nephropathy. (A, B) toluidine blue, ×200. Semi-thin section stained with toluidine blue for light microscopy. Light microscopic findings showed tubules with slight focal atrophy and loss, with interstitial fibrosis. (C, D) Electron microscopy ultrastructural examination showed small amounts of mesangial deposits. The glomerular basement membrane was normal in thickness, having partly irregular inner contours. Epithelial cell foot processes showed slight focal effacement (C: 7% uranyl acetate and 1% lead citrate 5,000×, D:7% uranyl acetate and 1% lead citrate 8,000×). (E, F) Immunofluorescence findings were consistent with immune-mediated nephropathy and suggestive of C1q nephropathy, with prominent mesangial C1q staining. (E) IgG showed 1 to 2+ diffuse segmental mesangial staining. (F) C1q deposition showed 2+ diffuse mesangial staining.
A cycle of methylprednisolone pulse therapy consisted of 20 mg/kg/day for 3 consecutive days, followed by 1 mg/kg per day of an oral steroid, such as deflazacort for 10 days. We tried three cycles and then tapered off for 3 months. After 3 months, routine urinalysis was persistently normal, with no hematuria or proteinuria for 6 months, and a follow-up biopsy was performed because urinary findings do not necessarily reflect kidney status in chronic glomerulonephritis after steroid therapy. Laboratory results were as follows: hemoglobin 13.7 g/dL, hematocrit 39%, white blood cells 4,400/mm3, platelets 252,000/mm3, serum total protein 6.7 gm/dL, albumin 3.9 gm/dL, cholesterol 150 mg/dL, triglycerides 85 mg/dL, aspartate aminotransferase 34 U/L, alanine aminotransferase 16 U/L, BUN 13.2 mg/dL, creatinine 9.53 mg/dL, C3 106 mg/dL, C4 12 mg/dL, and HBsAg (−). Urinalysis showed protein (−), glucose (−), RBC (−), and WBC 1+, a spot urine protein-to-creatinine ratio of 0.065, and eGFR 199 mL/min.
Up to 32 glomeruli were present in the follow-up biopsy (Fig. 2). The glomeruli were of normal or slightly increased size and mildly hypercellular, involving mesangial cells (Fig. 2C and 2D). Ultrastructurally, the glomerular basement membrane showed focal thinning but was normal in thickness, on average, having partly irregular inner contours (Fig. 2E and 2F). No electron-dense deposit was found. Epithelial cell foot processes showed focal slight effacement. Tubules revealed focal mild atrophy and loss with interstitial fibrosis (Fig. 2A and 2B). In comparison with the previous biopsy specimen, showing mesangial C1q and electron-dense deposits, they were no longer present in this one, suggesting healing of the C1q nephropathy.

Follow-up biopsy for C1q nephropathy. (A-D) H&E staining. Light microscopic findings showed glomeruli of normal or slightly increased size and mildly hypercellular, involving mesangial cells. Tubules revealed focal mild atrophy and loss with interstitial fibrosis (A: ×40, B-D: ×200). (E, F) Ultrastructurally, the glomerular basement membrane showed focal thinning but was normal in thickness, on average, with partly irregular inner contours (E: 7% uranyl acetate and 1% lead citrate 5,000×, F: 7% uranyl acetate and 1% lead citrate 6,000×).
DISCUSSION
C1q nephropathy was first described by Jennette and Hipp [1] in 1985. The diagnostic criteria for C1q nephropathy are dominant or codominant deposition of C1q (2+ or more on a scale of 0 to 4+) in the renal mesangium on immunofluorescence, with no clinical or serological evidence of lupus [1,2]. The prevalence of C1q nephropathy in renal biopsies ranges from 0.2% to 16% and it predominantly occurs in children [4].
The clinical features are diverse, ranging from asymptomatic hematuria or proteinuria to nephrotic syndrome with or without hypertension and renal insufficiency. The clinical outcomes are also varied. C1q nephropathy is usually considered to be a steroid-resistant nephrotic syndrome [1,5,7]. Despite immunosuppressive treatment, some cases present as rapidly progressive crescentic glomerulonephritis or as nephritic-nephrotic syndrome and eventually progress to end-stage renal disease, requiring renal replacement treatment [7-9].
C1q nephropathy is not always diagnosed with a first biopsy, for several reasons. First, the prevalence of C1q nephropathy is rare, and C1q immunostaining is not performed routinely at initial biopsies. Another cause could be a false-negative result in electron microscopy; here, the immunostaining showed an initial absence but subsequent increase and dominant, intense staining for C1q. We report a case with intermittent hematuria, first diagnosed as diffuse mesangial proliferative glomerulonephritis, but a follow-up biopsy showed C1q nephropathy, with C1q deposition in both immunohistochemistry and EM.
There are few reported studies in which outcomes in C1q nephropathy showed relationships with clinicopathological subsets. Vizjak et al. [9] reported that the outcome of C1q nephropathy varied according to the clinical presentation. They reported that C1q nephropathy showed a poor prognosis with nephrotic syndrome and FSGS. Furthermore, patients without glomerular abnormalities and nephrotic syndrome showed stable renal function even in the absence of treatment. However, Fukuma et al. [10] reported that there was no significant difference between histological findings and clinical outcomes among patients with no urinary abnormalities and those with nephrotic syndrome [7]. Fukuma et al. [10] also reported that mesangial C1q deposition had resolved in three patients at a second biopsy. One of them showed proteinuria and hematuria with no treatment; the others showed frequent relapses despite immunosuppressive treatment.
We treated C1q nephropathy with three cycles of methylprednisolone pulse therapy. After steroid therapy, the patient showed complete remission, with no proteinuria or hematuria over 6 months. A follow-up biopsy was performed and confirmed the absence of C1q deposition by both immunohistochemistry and EM on the biopsy. To our knowledge, this is the first reported case in South Korea of C1q nephropathy treated with methylprednisolone, in which complete remission was confirmed with a follow-up renal biopsy.