의사에게 요구되는 인간이해를 통합한 질병발생모델의 제안
Human Understanding is Expected of the Physician: Proposing a Model of Disease Development
Article information
Trans Abstract
In Harrison’s Principles of Internal Medicine, human understanding is emphasized as one of three necessary characteristics that a physician must have. Inflammation, which is caused by inflammatory inducers (inf-ids), is a fundamental feature of disease at the cellular and molecular levels. Inflammation protects the body, but excessive or prolonged inflammation can be damaging and can cause disease. Humans are repeatedly exposed to external and internal environmental factors that generate inf-ids throughout their lives. External environmental factors include microbial and non-microbial inf-ids, as well as stressors that inevitably arise during social interactions. Internal environmental factors include the adaptive physiological response that is present from birth. Inf-ids may also be produced by the four-step habit loop, which consists of a cue (e.g., stressor), emotions, routine act (adaptive response), and a reward. Immune cells in the circulatory system and in tissues may have positive and negative effects in inflammatory responses. However, low-grade inflammation may be difficult to detect. We propose a model of disease development that integrates external and internal environmental factors from the perspective of human understanding.
INTRODUCTION
Disease onset involves inflammation at the cellular and molecular levels [1-4]. Inflammation is mediated by the innate and acquired immune systems and it protects the body from infectious and non-infectious agents [1,2]. When the process progresses smoothly, inflammatory factors are removed by immune cells, and the damaged tissues are eventually repaired. However, if the inflammation is excessive or prolonged, cells and tissues can become damaged, resulting in various diseases [3,5,6]. Inflammation is an indispensable response that protects the human body, but it is also called the ‘silent killer’ because it can provoke disease [4].
The first page of Harrison’s Principles of Internal Medicine states that physicians require technical skill, scientific knowledge, and human understanding [7,8]. In addition to human understanding, Shakespearean breadth has now been introduced, emphasizing the need for a broader understanding of human beings as exemplified in the works of the great English playwright William Shakespeare [8]. Harrison’s Principles of Internal Medicine suggests that to understand the ultimate source of a disease and provide effective treatment, it is necessary to have detailed insight into the stressors that the patient has experienced, is currently experiencing, or expects to experience in the future, as well as the emotions induced by those stressors. It is important to understand that disease involves more than simply damage to the components of the human body.
In a previous report, we suggested that a human understanding of patients and diseases from the perspective of Shakespearean breadth is necessary to treat pancreaticobiliary disease, reduce the rate of recurrence, and ultimately prevent the disease [9]. The biomedical basis of human understanding has been linked to the habit loop and the adaptive response that are unconsciously and automatically activated by stressors [10-14]. Based on a previously proposed model of human understanding that was applied to pancreaticobiliary disease [9], we suggest a model of disease development that includes inflammatory mechanisms at the cellular and molecular levels.
DISEASE AND INFLAMMATION
Diseases are conditions in which cells fail to maintain homeostasis due to molecular or structural alterations and damage caused by physical and chemical stimuli [2,15]. Various stimuli can damage cells and activate inflammatory pathways, which have four components: inducers, sensors, mediators, and target tissues (Fig. 1) [2,16-19]. Inducers stimulate the sensory receptors of immune cells, induce the secretion of inflammatory mediators (IMs), and may be classified as microbial or non-microbial [19]. Non-microbial inducers may be further classified as exogenous substances of inducers (Exo-SIs), which are introduced into the body from an external source, or endogenous substances of inducers (Endo-SIs), which are generated inside the body.
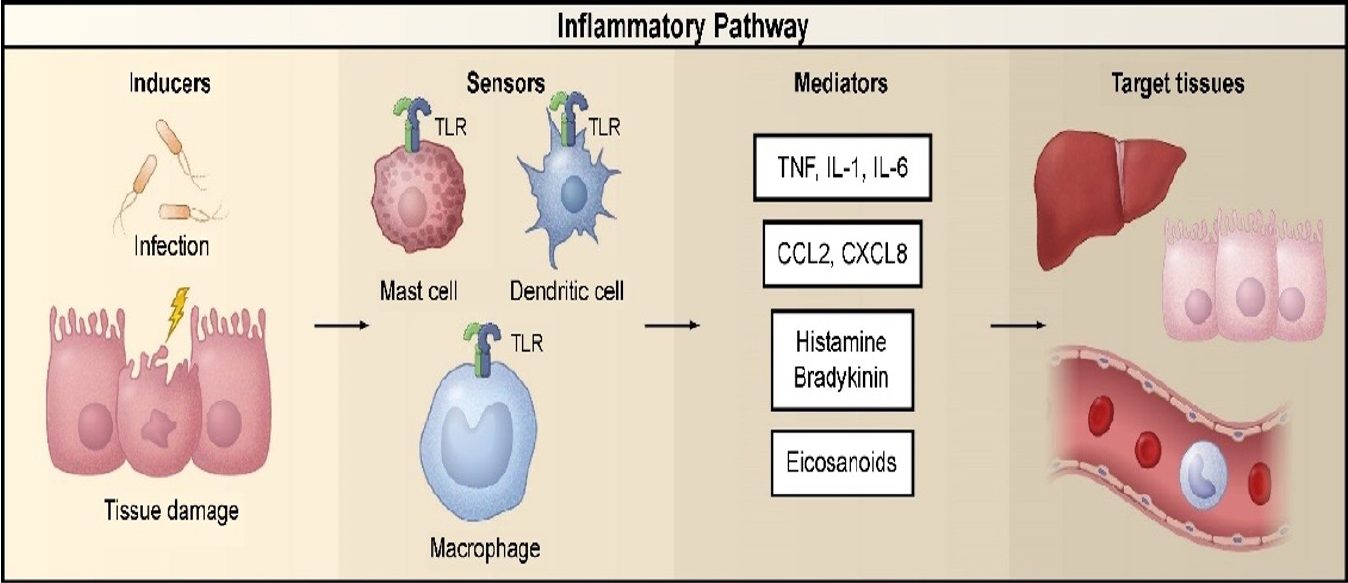
Inflammatory pathway components. The inflammatory pathway consists of inducers, sensors, mediators, and target tissues. Inducers initiate the inflammatory response and are detected by sensors. Sensors, such as TLRs, are expressed on specialized sentinel cells, such as tissue-resident macrophages, dendritic cells, and mast cells. They induce the production of mediators, including cytokines, chemokines, bioactive amines, eicosanoids, and products of proteolytic cascades, such as bradykinin. These inflammatory mediators act on various target tissues to elicit changes in their functional states that optimize adaptation to the noxious condition (e.g., infection or tissue injury) associated with the particular inducers that elicited the inflammatory response. The specific components shown represent only a small sample of the myriad different sensors, mediators, and target tissues involved in the inflammatory response. Reproduced from Medzhitov [2]. TLR, Toll-like receptor; TNF, tumor necrosis factor; IL, interleukin; CCL2, C-C motif chemokine ligand 2; CXCL8, C-X-C motif chemokine ligand 8.
Exo-SIs generally enter the body through the nose, mouth, or skin [1]. Some Exo-SIs are harmful in any quantity, such as tobacco, air pollutants, asbestos fibers, crystalline silica, caustic substances, paraquat, coal tar, defoliants, perfluorooctanoic acid, polyethylene, and polyvinyl chloride [20-28]. Other Exo-SIs, including food and drink, may only be harmful if the body ingests more than it can process; this can result in excessive production of substances, such as cholesterol crystals, lipids, glucose, urate crystals, and acetaldehyde. Hyperlipidemia, hyperglycemia, hyperuricemia, or excessive production of aldehyde can stimulate monocytes, macrophages, endothelial cells, smooth muscle cells, mast cells, neutrophils, hepatocytes, Kupffer cells, and hepatic stellate cells, leading to the production of IMs [18,29-32]. In addition, high-fat and/or high-carbohydrate intake can elevate the levels of endotoxins, which are potent inflammatory antigens, in the blood [33-35].
Food may also be considered an Exo-SIs when consumed excessively. The lifestyles of many people have changed, and levels of physical activity have decreased due to the mechanization of labor and transport. Consequently, calorie intake is often excessive, and obesity has become more prevalent [36-39]. Obesity results in chronic low-grade inflammation (LGI) via the following mechanisms: dysregulated secretion of adipokines; secretion of cytokines and chemokines from macrophages, endothelial cells, and other immune cells in adipose tissue; cell death and hypoxia in adipose tissue; an increase in intestinal lipopolysaccharide-containing microbiota; and a decrease in the activity of the vagus nerve, which is a vital component of the inflammatory reflex [36,38-40].
Endo-SIs include stress hormones, pathogenic substances, physiological secretions (e.g., gastric acid, pancreatic juice, and bile), and metabolites (e.g., urine, stool, and sweat). Stress hormones are generated via the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS), which are activated by physical or psychological stressors [11,12,41,42]. When the HPA axis is activated, corticotropin-releasing factor is secreted from the hypothalamus, adrenocorticotropic hormone is released from the pituitary gland, and gucocorticoids (GCs) are secreted from the adrenal cortex. Stimulation of the somatic nervous system by stressors induces the secretion of norepinephrine (NE) from the terminal nerve and epinephrine (EP) from the adrenal medulla via the pons and spinal cord. NE, EP, and GCs bind to receptors on immune cells (e.g., neutrophils, monocytes, natural killer cells, macrophages, T cells, and B cells) in the blood, tissues, and organs (e.g., bone marrow, thymus, spleen, gut-associated lymphoid tissue, bronchus-associated lymphoid tissue, and lymph nodes); this activates intracellular signaling pathways and produces various IMs [3,11,13,43-45].
Pathogenic substances are associated with necrotic cell death and tumors. Three types of pathogenic substance are associated with necrotic cell death [46]. The first type includes chromatin-associated high-mobility group box 1 proteins, heat shock proteins, and purine metabolites (e.g., adenosine triphosphate and uric acid); these are released from cells after the plasma membranes are ruptured. The second type includes hyaluronan, heparan sulfate, and biglycan, which are produced after the extracellular matrix is degraded. The third type includes the biologically active pro-inflammatory cytokines and chemokines present in cells, including interleukin (IL)-1a and IL-33.
Pathogenic substances are also secreted by tumors or induced by cancer therapy. The tumor microenvironment consists of inflammatory cells that stimulate tumor proliferation and migration [47-50]. Tumor cells secrete cytokines and chemokines to attract leukocytes, such as neutrophils, dendritic cells, macrophages, eosinophils, mast cells, and lymphocytes. These leukocytes produce cytokines and cytotoxic mediators to further promote inflammation. Cancer therapies include chemotherapy, radiotherapy, and immunotherapy; these treatments kill tumor cells, releasing substances that stimulate inflammation [51,52].
The other Endo-SIs include enzymes (e.g., in saliva, gastric acid, pancreatic juice, and bile) and metabolites (e.g., in urine, stool, and sweat) that are involved in normal physiological and metabolic processes. These play roles in digestion and maintain balance in the internal environment [53]. However, there are two situations in which these can become Endo-SIs and cause inflammation. This may occur if the site of action is altered. For example, gastric acid and pepsin facilitate the digestion of food but can cause inflammation in the esophageal epithelium if they are refluxed into the esophagus [54,55]. In the congenital anomaly of pancreaticobiliary maljunction, digestive pancreatic juice is released into the biliary tract causing inflammation [56]. Inflammation can also occur if gastric acid, bile, pancreatic juice, or stool is released into the abdominal, retroperitoneal, or thoracic cavities following gastroduodenal or intestinal perforation or pancreatitis [57,58]. The other situation in which enzymes or metabolites can become Endo-SIs is when their excretion is impaired. Bile accumulates in the biliary tract or blood when it cannot be secreted into the duodenum due to hepatitis, gallbladder dyskinesia, sphincter of Oddi dysfunction, or a biliary obstruction. This may cause cholecystitis, hepatitis, neurotoxicity, or systemic inflammatory response syndrome [59-61]. Furthermore, various products of protein metabolism are excreted in the urine. If renal failure inhibits the excretion of these products, they accumulate in the blood and become inflammatory inducers (inf-ids) that stimulate systemic inflammation [62,63].
External environmental and internal human characteristics that induce and prolong inflammation
People are exposed to Exo-SIs and microbial inducers throughout their lifetimes [64]. They frequently eat, drink, and inhale Exo-SIs (e.g., food, cigarettes, alcohol, and air pollutants) for survival or pleasure, or even unconsciously. In particular, humans have an inherent fear of starvation and tend to overeat unknowingly [65]. In addition, microbes are always present both inside and outside the human body [1].
Psychological stress can produce Endo-SIs at various times of life [66-69]. As seen in the many plays written by William Shakespeare, stress results from conflicts when people with different backgrounds, cognitive biases, values, and goals interact. Moreover, stressors are natural and inevitable in human life. Humans have evolved the adaptive response to situations in which survival or self-esteem is threatened [11,12,70,71]. This adaptive response includes a physical (behavioral) reaction that activates the skeletal muscles and a physiological reaction that activates the internal organs [11,72,73]. These reactions are stimulated by emotions that occur when a particularly stressful situation is encountered [11,13,70,72-74]. Cannon reported that the physical fight-or-flight response is activated by anger or fear, with fighting being a response to anger and flight a response to fear [70]. Among the stress hormones that can function as Endo-SIs (EP, NE, and GCs), catecholamine (EP and NE) plays a key role in the fight-or-flight response [11,45,70,75]. Interestingly, the HPA axis and SNS are always activated simultaneously and produce stress hormones [11,41-43,45]. Thus, anger and fear stimulate the fight-or-flight response and the physiological reaction. Likewise, other emotions, such as despair, depression, sadness, or anxiety can also trigger the adaptive response [11-13,69].
Emotions are often responsible for human behaviors [76-79]. Emotions also synchronize those behaviors that introduce Exo-SIs into the human body, such as overeating, alcohol consumption, and smoking [80]. In addition, emotions may affect food choices and eating patterns. Strong emotions may inhibit food intake, but less intense emotions may have unpredictable effects on eating habits [81,82]. Sometimes, sweets and high-calorie foods are consumed in response to negative emotions. One study reported a 32% higher prevalence of depression among people who were overweight or obese compared to people of normal weight [83].
Smoking and excessive alcohol consumption may sometimes calm negative emotions [84-86]. According to a segmentation study in 1981, smoking may relieve anxiety, depressive symptoms, and stress; it may also improve self-control [87]. The tension reduction theory suggests that alcohol consumption affects the central nervous system and reduces stress [88].
Interestingly, the adaptive response is also activated by perceived threats [11,13,44,89,90]. Therefore, regardless of authenticity, if a situation is perceived as threatening, the adaptive response is triggered automatically. Furthermore, even in a genuinely threatening situation, the adaptive response may vary depending on the appraisal of the situation [11,75,89]. In fact, the adaptive response occurs only when the individual perceives and appraises a situation as a threat to survival. Finally, the adaptive response can be triggered by recollection of past events or anxiety about the future [11,12,79,91-93].
Past experiences that are associated with pain or fear, such as childhood trauma, sexual assault, war, traffic accidents, or life-threatening situations can cause schizokinesis and may be stored in long-term memory [94-96]. As demonstrated by Pavlov’s conditioned reflex, the recall of invisible memories can activate a visible physiological response (e.g., saliva) [97]. Traumatic experiences in childhood can alter stress sensitivity, triggering the adaptive response under relatively low levels of stress [91,98-103]. Traumatic childhood experiences can also result in chronic activation of the adaptive response for decades, repeatedly generating Endo-SIs [102,104]. In addition, very severe childhood trauma may increase the frequency of Exo-SIs production and exacerbate problems (e.g., with alcohol consumption, smoking, physical inactivity, drug abuse, or obesity) [99,105].
Therefore, systems that are designed to maintain homeostasis can damage the human body and cause disease [2,4,11,12]. As described by Cannon, emotions have evolved to trigger and control the fight-or-flight response, which is essential for survival [70,106]. Such emotions can also maintain homeostasis and they are key components in the four-step habit loop for coping with stressors [9,77,107]. The adaptive response operates automatically in life-threatening situations [11,12,75]. Immune cells play pivotal roles in eliminating pathogenic inducers [4,108,109]. These internal systems protect the human body from stressors, Exo-SIs, and Endo-SIs. However, inappropriate, excessive, or prolonged activation of these systems can damage the human body [2-6,11,12,17,19,107-109].
Inf-ids may be repeatedly introduced from outside the human body or produced inside the body due to habit loops that are initiated by stressors [9,10,14,110]. Habit loops are behavioral patterns that operate reflexively in response to similar situations. When emotions are triggered by stressors, the adaptive response may be activated immediately. Consequently, Exo-SIs are introduced into the body using the skeletal muscles as an alternative behavioral pattern to the fight-or-flight response; the physiological response may also be activated by stress hormones. Subsequently, emotional tension is temporarily relieved, and there is a psychological reward. Although this psychological compensation is temporary, individuals will tend to routinely repeat the same behavioral pattern when they encounter similar situations to achieve psychological relief [9,10,14,110]. Therefore, familiar stressors create a four-step habit loop involving a cue (e.g., stressor), emotions, routine act (adaptive response), and a reward [9].
A chronic protective inflammatory response can result in disease. This may occur when inf-ids persist and the inflammatory response is lower than normal [1,2,111,112]. Pathogenesis can also develop if the link between the function of the systemic anti-inflammatory response and the diffusible neural network that controls the spread of inflammation is disrupted [111,112]. Changes in the pattern of disease can also lead to persistent inflammation. In the past, many diseases were caused by acute inflammation due to infections or injuries. However, most chronic inflammatory diseases are now due to excessive food or alcohol consumption, tobacco, air pollutants, or psychosocial stress [2,15,16]. A key characteristic of chronic inflammatory diseases is the presence of LGI, in which typical inflammatory symptoms (e.g., pain, fever, erythema, and swelling) are not evident [2]. LGI involves only a two- to four-fold increase in the levels of inflammatory markers such as cytokines and acute phase proteins [113-115]. LGI differs from that observed in typical acute inflammation because the level of inflammation is relatively low and it is due to other than an infection or tissue injury. In addition, the inflammation is systemic rather than localized and occurs over a longer period [16]. Pain, warmth, swelling, and erythema can be used to recognize and treat acute inflammation but LGI is more difficult to detect. However, if LGI persists, it may result in damage to cells and loss of organ function. LGI is characteristic of chronic diseases and is often associated with aging, hypertension, coronary heart disease, type 2 diabetes, metabolic syndrome, chronic obstructive pulmonary disease, osteoarthritis, post-traumatic stress disorder, depression, autoimmune diseases, functional dyspepsia, atriall fibrillation, and cancer [5,64,113-133].
A model of disease development that integrates human understanding
Figure 2 shows a model of disease development that integrates human understanding and involves external environmental and internal human characteristics, as described in the previous section. Stressors formed by memories, present situations, or future concerns activate the four-step habit loop. Emotions are elicited after the stressor is perceived and routine act (adaptive response) is triggered. The emotional tension is temporarily relieved, and a psychological reward is obtained. Individuals routinely repeat the four-step habit loop whenever they encounter similar stressors.
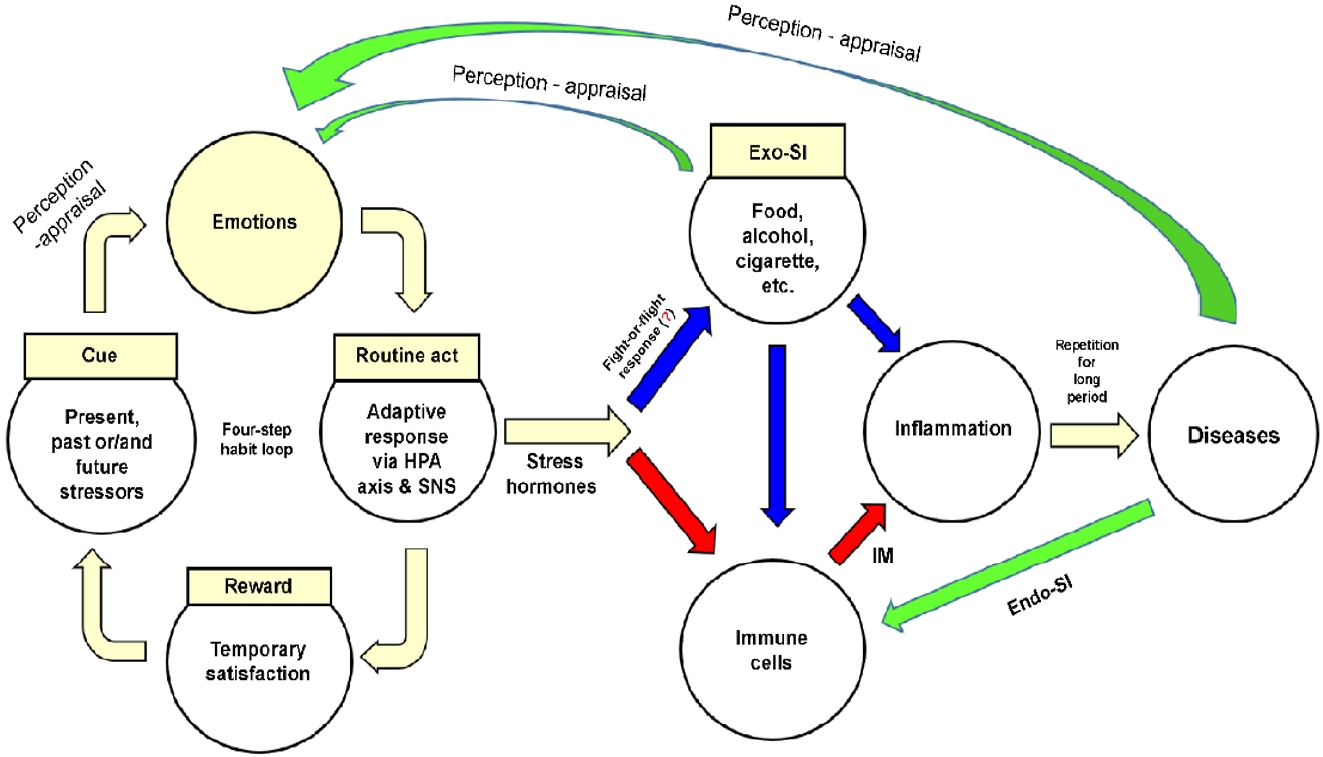
A model of disease development that integrates human understanding. Stress hormones mean glucocorticoids-epinephrinenorepinephrine. Exo-SI, exogenous substance of inducer; HPA, hypothalamic-pituitary-adrenal; SNS, sympathetic nervous system; IM, inflammatory mediator; Endo-SI, endogenous substance of inducer.
The adaptive response of the four-step habit loop immediately activates the HPA axis and SNS, having two effects. First, the skeletal muscles are stimulated to introduce Exo-SIs (e.g., food, alcohol, or tobacco) into the body. However, excessive introduction of Exo-SIs may cause inflammation of the target tissue or produce IMs by stimulating immune cells in the blood or tissues. Second, a physiological reaction (involving GCs from the HPA axis or NE and EP from the SNS and adrenal medulla) produces IMs by stimulating immune cells. The IMs together with recruited immune cells cause inflammation in the target tissues. When inflammation is repeatedly triggered by Exo-SIs and/or Endo-SIs over a prolonged period, the target tissues are unable to recover and cells become irreversibly damaged. Eventually, organ function is impaired and disease develops.
Chronic inflammation becomes part of a vicious cycle before and after disease development. First, emotions elicited when Exo-SIs are introduced into the body reactivate the four-step habit loop [134]. Second, the presence of disease is perceived as a threat, inducing negative emotions such as despair, fear, and anger [69,135], this also activates the four-step habit loop. Third, tissue necrosis due to the disease, substances secreted by tumor cells (e.g., cytokines and chemokines), and substances that have accumulated due to the disease (e.g., bile, pancreatic juice, urine, and urea) stimulate immune cells and exacerbate inflammation.
LIMITATIONS OF THE MODEL
This model has some limitations. First, the model is applicable only to diseases caused by repeated overstimulation of inf-ids over a prolonged period and may not apply to diseases caused by nutrient deficiencies. Second, the model may be less applicable to diseases that do not involve the four-step habit loop. Although diseases caused by congenital chromosomal abnormalities are due to an excess or deficiency of critical substances, they are not caused by stressors and emotions but rather by chromosomal alterations. Similarly, the model may not be applicable to diseases caused by infections because here the relationship between disease and the four-step habit loop is weak except infections associated with compulsive sexual behavior and injection drug use [136-139]. Third, the model is not suitable for understanding the impact of involuntary Exo-SIs, such as fine-particulate air and water pollutants produced in the individual’s occupational environment or by industry or traffic (e.g., lead, diesel, tire particles, asbestos fibers, crystalline silica, defoliants, perfluorooctanoic acid, polyethylene, and polyvinyl chloride). Fourth, even when stressors, Exo-SIs, and/or Endo-SIs are similar, not all individuals will develop the disease, or the same disease even if they do. Such a phenomenon is also difficult to explain with this model. The incidence of disease may vary depending on individual differences, such as in the ability to perceive stressors, physical health, muscle volume, telomere length, dehydration, and microbiota [11,140-144]. For example, the same stressors may elicit different negative emotions in different individuals [11,69,145]. Essential factors of the pathogenesis of diseases usually include environmental and genetic factors [65,73,146]. In addition, personal factors, such as perception and interpretation abilities, emotion control, and/or the four-step habit loop may have an important impact on pathogenesis [11,69,75,96,145,147]. Fifth, chronic disease development is frequently associated with LGI [113-133]. However, recognizing LGI is difficult because the signs associated with acute inflammation are absent or difficult to detect. Our model does not explain why the cellular damage that leads to disease cannot be detected early, and further studies on this topic are needed. Furthermore, diagnostic markers and tools that can detect LGI, such as the high-sensitivity C-reactive protein test, are required [148,149].
CONCLUSIONS
The requirement for human understanding means that physicians need an awareness of the external environment, as well as internal structural and functional characteristics, to monitor disease progression and provide effective treatments [9,69,91,96]. The human body is not sufficiently robust to withstand ongoing inflammation over a prolonged period, and individuals are constantly exposed to microbial and non-microbial inducers that can cause inflammation throughout their lives. Individuals have both adaptive and innate immune systems from birth, whereas the four-step habit loop and acquired immune system develop gradually thereafter. These function as protective systems that maintain homeostasis and promote survival against a background of changes in the external and internal environments. However, paradoxically, these systems may also damage the human body if they do not develop correctly or are activated for a prolonged period. They may be activated unconsciously in response to current situations but can also be triggered by recollection of past events or anxiety about the future. Importantly, the LGI that is frequently associated with chronic disease is difficult to detect.
Diseases are not simply disorders of the structural components of the human body but are also influenced by stressors experienced in the past or present, or anticipated in the future. Diseases may result from the interaction between genetic and environmental factors. Furthermore, how an individual perceives and interprets stressors, controls his or her emotions, and adjusts his or her four-step habit loop may be crucial.
The priority in treatment is recovery from the ill health caused by stimulation with microbial or non-microbial inducers. From the perspective of human understanding, treatment may be enhanced by increasing the patient’s awareness of the four-step habit loop because this mediates the repeated ingestion of Exo-SIs and/or produces Endo-SIs in response to stressors. Treatments should also be designed to reduce the likelihood of disease recurrence. Furthermore, disease development may be prevented by understanding how stress results from conflict when people with different values and goals interact, similar to the characters described in the many plays written by William Shakespeare. In conclusion, a disease development model that embraces the internal and external environmental characteristics described above is needed to prevent and treat disease. Therefore, we present a model of disease development that integrates human understanding and involves these characteristics.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This review was funded by the Soonchunhyang University Research Fund.
AUTHOR CONTRIBUTIONS
Conceptualization: S.H.P; Methodology: H.A.L; Software: S.P, J.Y.K; Validation: S.W.C, S.B.B, T.H.L, J.H.M; Investigation: S.H.P, H.A.L; Resources: S.H.P, S.H.J; Writing - original draft preparation: S.H.P, S.P, J.Y.K; Writing - review and editing: J.Y.K, S.P, S.H.P; Visualization: S.M.L; Supervision: S.H.P; Project administration: S.H.P.
Acknowledgements
The authors would like to thank Shi Nae Yu, M.D., Division of Infectious Diseases, Department of Internal Medicine, Sae Hwan Lee, M.D., Division of Gastroenterology, Department of Internal Medicine, Young Sin Cho, M.D., Division of Gastroenterology, Department of Internal Medicine, and Jin Myoung Seok, M.D., Department of Neurology, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea, for recommending references.