|
 |
| Korean J Med > Volume 100(3); 2025 > Article |
|
Abstract
Chronic kidney disease (CKD) affects approximately 10-15% of adults globally and is a significant public health issue owing to its association with cardiovascular disease, end-stage kidney disease, and high healthcare costs. Hyperuricemia has emerged as an important modifiable risk factor influencing CKD progression. Elevated uric acid (UA) levels contribute to kidney injury through crystal-dependent mechanisms, including monosodium urate crystal deposition and NLRP3 inflammasome activation, and crystal-independent pathways, such as endothelial dysfunction, activation of the renin-angiotensin-aldosterone system, and oxidative stress. Observational studies have consistently linked hyperuricemia to an increased risk of CKD onset and accelerated disease progression. Nevertheless, randomized controlled trials and meta-analyses investigating UA-lowering therapy (ULT) for asymptomatic hyperuricemia have yielded conflicting results regarding its effectiveness in slowing CKD progression. Clinical guidelines also differ: Japanese guidelines recommend ULT for serum UA levels exceeding 8.0 mg/dL, whereas Western guidelines generally do not support routine treatment of asymptomatic hyperuricemia. Thus, there remains a clear need for large-scale, long-term studies to define patient subgroups most likely to benefit from ULT and guide individualized treatment approaches.
Chronic kidney disease (CKD) affects approximately 10-15% of adults worldwide and imposes a major public health burden because of its association with cardiovascular events, end-stage kidney disease (ESKD), and elevated healthcare costs [1-3]. To address this burden, interventions aimed at factors that trigger or worsen CKD would be required; hyperuricemia is one such factor [4].
Although high purine intake and alcohol consumption commonly lead to hyperuricemia, its prevalence has increased due to increased fructose consumption, obesity, metabolic syndrome, and the use of certain medications [5]. Although hyperuricemia is a key risk factor for gout, many patients remain asymptomatic. Reduced uric acid (UA) excretion can elevate serum levels in patients with CKD, potentially causing chronic inflammation and tissue injury via monosodium urate (MSU) crystal deposition [6].
Observational research suggests that hyperuricemia can independently increase the likelihood of CKD onset and progression. However, large-scale randomized controlled trials (RCTs) investigating asymptomatic hyperuricemia have yielded mixed results regarding whether lowering UA levels can effectively slow the course of CKD [7,8]. Such inconsistencies have prompted a debate on whether hyperuricemia serves as a direct pathogenic factor or merely reflects broader metabolic disturbances.
The current review aimed to summarize the key pathophysiological mechanisms linking hyperuricemia to CKD, highlighting recent clinical evidence and providing an evidence-based framework for managing asymptomatic hyperuricemia in routine practice.
UA, the final product of purine metabolism, is predominantly synthesized in the liver. Approximately 70% of UA is excreted by the kidneys, whereas the remaining 30% is excreted through the intestines [9]. Purines originate from endogenous molecules (RNA, DNA, and ATP) or dietary sources (e.g., meat and fructose). In recent years, the increased intake of purine-rich foods, alcohol, and fructose combined with obesity, CKD, and certain medications has contributed to a steady increase in hyperuricemia [10,11].
Purine catabolism proceeds via two main pathways, adenine and guanine (Fig. 1). Adenosine monophosphate (AMP) and guanosine monophosphate are first converted to adenosine and guanosine by nucleotidase enzymes, and then further degraded to xanthine, which is subsequently converted to UA by xanthine oxidase. Most mammals (except humans and higher primates) and amphibians possess uricases to oxidize UA into allantoin, thereby avoiding a significant elevation of UA in the serum. Humans lack functional uricases, making them more susceptible to hyperuricemia [12].
There are notable sex-related differences in the UA metabolism, with men generally exhibiting higher serum UA levels than women. These differences can be attributed to various factors, including body composition. Men tend to have a larger body size and greater muscle mass, leading to increased purine turnover and UA production. Hormonal influences also contribute to the uricosuric effects of estrogen in women [13].
UA is a significant antioxidant, accounting for approximately half of the total antioxidant capacity of blood [14]. It may also play a role in the regulation of blood pressure under low-sodium conditions. Additional evidence suggests that it might as well protect against cancer and neurodegenerative diseases [15,16].
Serum UA concentrations roughly above 6.8 mg/dL is its solubility threshold, raising the risk of MSU crystal formation [17]. Hyperuricemia, often defined as > 7.0 mg/dL in men or 6.0 mg/dL in women [18], is strongly linked to gout besides being associated with cardiovascular disease, kidney disease, and metabolic syndrome [4,19]. Asymptomatic hyperuricemia without overt gout or nephrolithiasis is associated with a higher risk of cardiovascular disorders and CKD [20].
Hyperuricemia-related renal injury involves both crystal-dependent and crystal-independent mechanisms (Fig. 2).
Persistently elevated serum UA levels can result in the formation of MSU crystals that are deposited in the joints, blood vessels, and renal tissue [21-23]. MSU crystals bind toll-like receptor 2/4 on macrophages, triggering myeloid differentiation primary response 88 nuclear factor kappa B (NF-κB) signaling and inducing NOD-like receptor (NLR) family pyrin domain containing 3 (NLRP3) and pro-interleukin (IL)-1β expression (Fig. 3) [24]. Since macrophages engulf these crystals, heightened nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, cathepsin B leakage, and intracellular K+ depletion collectively activate the NLRP3 inflammasome [25]. Once active, the latter cleaves pro-IL-1β and pro-IL-18 into mature forms and splits gasdermin D, promoting pyroptosis and the release of inflammatory mediators [26].
In the kidney, MSU crystal deposition triggers the overactivation of inflammasomes in the macrophages and neutrophils, elevating IL-1β and other cytokines [27,28]. Chronic urate nephropathy arises from granulomatous macrophage infiltration and persistent inflammation, culminating in tubular obstruction, interstitial fibrosis, and crystal cast formation at low pH [29-31]. Imaging studies have linked MSU crystals in blood vessels to accelerated atherosclerosis, suggesting similar risks of asymptomatic hyperuricemia [32].
Some studies have indicated that the effects of hyperuricemia on CKD extend beyond crystal deposition [33,34]. Soluble UA can contribute to CKD progression via endothelial dysfunction, a heightened renin-angiotensin-aldosterone system (RAAS), and oxidative stress (Fig. 4). Hyperuricemia reduces nitric oxide synthase availability by inhibiting endothelial nitric oxide synthase, elevating vascular resistance, and promoting glomerular hypertension and tubulointerstitial damage [35]. Simultaneously, RAAS activation results in a hypertensive environment, while increased aldosterone and sodium reabsorption exacerbate glomerular strain [34,36-38].
Soluble UA can further stimulate inflammatory pathways, including mitogen-activated protein kinases (MAPK) and cyclooxygenase-2, leading to the epithelial-mesenchymal transition in tubular cells, which is a key step in renal fibrosis [33,39,40]. Furthermore, UA elevates reactive oxygen species through NADPH oxidase, activating NLRP3 and NF-κB, and promoting chronic inflammation even without crystals [41-43]. This environment fosters mitochondrial damage and the release of cytokines like IL-1β and tumor necrosis factor-α, accelerating fibrosis [44-46]. Emerging data suggests that soluble UA inhibits AMP-activated protein kinase, drives mammalian target of rapamycin activation, and undermines autophagy, thereby exacerbating tubular cell damage [47,48].
Several epidemiological studies and meta-analyses have indicated that serum UA levels strongly influence the incidence and progression of CKD. In a meta-analysis of 15 cohort studies (n = 99,205), each 1 mg/dL rise in UA increased CKD risk by 22%, notably among those under 60 years of age [49]. Another analysis of 190,718 individuals concluded that hyperuricemia was an independent predictor of CKD onset, especially in those without preexisting kidney disease [50]. A recent pooled analysis of 24 cohort studies (n = 412,238) found that a lower UA correlated with a relative risk of 0.44 compared to the highest UA group, suggesting significant renal protection [51]. Similarly, multiple long-term cohorts showed that elevated UA levels independently predicted estimated glomerular filtration rate (eGFR) decline [52].
Hyperuricemia is more common in patients with advanced CKD than in the general population, reaching 40-80% in some studies [53]. A meta-analysis of 23 studies (212,740 individuals) reported an overall hyperuricemia prevalence of 43.6% in patients with CKD, with higher rates in men (67.4%) than in women (32.6%) [54]. Similarly, a study conducted in Bangladesh reported that 23.2% of men and 14.6% of women with CKD have hyperuricemia [55].
In contrast, a cohort study in Taiwan demonstrated that hyperuricemia was associated with a higher odds ratio for new-onset CKD in women (3.813) than in men (1.989), suggesting a greater risk in women [56]. Additionally, each 1 mg/dL increase in serum UA was linked to a higher hazard ratio for renal function deterioration in women than in men, indicating that rising UA levels may have a more pronounced impact on CKD progression in women [57].
Although observational studies have consistently linked hyperuricemia to CKD progression, evidence from Mendelian randomization (MR) studies has generated controversy regarding the causality. MR studies utilizing genetic variants as proxies failed to conclusively demonstrate a causal relationship between serum UA levels and CKD incidence or eGFR decline [58,59]. Furthermore, MR evidence regarding cardiovascular and hypertensive outcomes contradicts findings from observational research, raising further debate over whether hyperuricemia directly contributes to kidney damage or merely serves as a marker of broader metabolic dysfunction [60].
These conflicting findings highlight the complexity of the decision to initiate UA-lowering therapy (ULT) in patients with CKD. Although many RCTs have confirmed that ULT reduces serum UA levels, its overall impact on CKD progression remains controversial (Table 1). Some studies have suggested that allopurinol can slow CKD progression and lower cardiovascular risk [61], whereas topiroxostat and febuxostat have been associated with decreased proteinuria or slower eGFR decline [62,63].
The PERL trial in patients with type 1 diabetes and the CKD-FIX trial in patients with stage 3-4 CKD revealed that allopurinol did not demonstrate a significant benefit over placebo in preventing CKD progression [7,8]. These studies included populations whose mean UA levels were inconsistent with remarkable hyperuricemia, raising questions regarding their generalizability to real-world patients with more severe hyperuricemia [8]. Both the PERL and CKD-FIX included a substantial number of patients with normal serum UA levels and used intention-to-treat analyses, including those who discontinued treatment because of adverse events or non-adherence. Notably, approximately 17.5% to 30.0% of the participants dropped out before completing the study, which could have affected the final results. Nata et al. [64] also reported that febuxostat lowered serum UA levels but failed to remarkably improve eGFR.
Meta-analyses yielded mixed outcomes; one found no clear benefit regarding major events or renal failure [65], whereas others noted improved renal function with allopurinol or febuxostat [66]. Long-term studies appeared more favorable, reporting a slower eGFR decline [67,68], although questions regarding dose dependence and agent selection persisted [69].
Overall, the varied outcomes suggest that CKD risk level, treatment duration, and ULT choice all modulate potential gains. Large-scale, long-term RCTs that stratify patients by renal function and additional risk factors are required to identify patients who benefit the most.
Lifestyle modifications play a crucial role in the management of hyperuricemia. The American College of Rheumatology recommends limiting the intake of alcohol, purine-rich foods, and high-fructose corn syrup while encouraging weight loss [70]. A study conducted in Japan revealed that adherence to dietary and lifestyle recommendations before starting medication led to a 7.8% reduction in serum UA (SU) levels over a 6-month period [71].
Alcohol consumption increases the risk of gout in a dose-dependent manner, with beer and distilled spirits exerting particularly significant effects on SU levels [72,73]. Moreover, fructose intake, commonly through sweetened beverages and fruit juices, has been associated with an increased risk of hyperuricemia and gout flare-ups, highlighting the importance of limiting consumption [74].
Obesity is an important risk factor for gout. An increase in body mass index (BMI) > 5% may result in a 60% increase in the frequency of gout attacks, whereas a 5% decrease in BMI can lower this risk by approximately 40% [75]. Maintaining adequate hydration and, if necessary, using alkalinizing agents (e.g., sodium bicarbonate) can help prevent the formation of UA stones in acidic urine. However, in patients with CKD, lifestyle modifications alone may be insufficient to manage hyperuricemia effectively, as these patients often have impaired urate excretion [76].
The initiation of ULT in patients with CKD requires careful dose selection and monitoring. Xanthine oxidase inhibitors are a primary class of drugs, and allopurinol is commonly used because of its effectiveness and affordability. In patients with CKD, however, therapy starts at a low dose (e.g., 50-100 mg/day) and is titrated slowly while monitoring for hypersensitivity and renal function changes. Rare, but severe, allopurinol hypersensitivity syndrome may occur, especially in patients with renal impairment or specific ethnic backgrounds. Screening for HLA-B*58:01 is recommended for high-risk groups (e.g., Koreans and Chinese Han) [18].
Febuxostat, a selective xanthine oxidase inhibitor, lowers serum UA levels more effectively than allopurinol. Although the CARES study reported increased cardiovascular mortality in patients with gout and cardiovascular disease [77], subsequent trials (e.g., FAST study) demonstrated its non-inferiority to allopurinol [78,79]. In patients with advanced CKD, febuxostat may be preferred when the use of allopurinol and uricosuric agents is limited.
Probenecid, benzbromarone, and lesinurad increase renal UA excretion but have limited efficacy, low glomerular filtration rate, and potential toxicity [36]. Rasburicase and pegloticase are restricted to severe hyperuricemia or tumor lysis syndrome. Certain angiotensin II receptor blockers or sodium glucose cotransporter 2 inhibitors may also indirectly reduce UA [80].
Although the cost-effectiveness of ULT in patients with CKD remains controversial, studies primarily focusing on gout have consistently highlighted the efficacy and affordability of allopurinol. Nonetheless, in patients with CKD, initiating allopurinol treatment at a low dose (e.g., 50-100 mg/day) with close monitoring of hypersensitivity and renal function changes is critical. Considering that approximately 12% of Koreans carry the HLA-B58:01 allele, screening is recommended to reduce the risk of severe hypersensitivity reactions [81]. Furthermore, specific evaluations suggested that even after accounting for the expense of HLA-B*58:01 screening, febuxostat may be comparatively cost-effective in patients with CKD [82].
The potential for severe skin reactions and other complications, which may be more pronounced in patients with CKD, highlights the need for careful patient selection and vigilance when using allopurinol [83]. Ultimately, a personalized approach that considers each patient’s risk factors and the distinct characteristics of the available medications is crucial for choosing the most suitable urate-lowering therapy.
Research on treatment adherence among individuals with asymptomatic hyperuricemia is sparse, and much of the available information is indirectly obtained from studies involving patients with gout. Generally, the adherence to ULT in the aforementioned patient population remains poor and varies considerably worldwide, ranging from 17% to 78% [84,85]. In Korea, data from the Health Insurance Review and Assessment Service between 2007 and 2015 showed that although over 80% of patients with gout received at least one ULT prescription, only 35% persisted with the medication for more than 5 years [86]. Similarly, large-scale retrospective analyses have found that more than half of patients with gout who initiated allopurinol therapy discontinued treatment, and adherence significantly impacted the achievement of target serum UA levels: only 22.5% to 27.8% of low-adherence patients reached the target (< 6 mg/dL), compared to 49.3% to 56.8% of high-adherence patients [87]. Moreover, the CARES study reported that approximately half of the patients with gout at elevated cardiovascular risk ceased trial medications, complicating efficacy assessments [77].
Factors contributing to low adherence in patients with gout include symptom improvement, concerns about side effects, and financial burdens. Given that patients with CKD and asymptomatic hyperuricemia do not experience acute symptoms, such as painful gout attacks, they may feel less compelled to continue treatment, potentially resulting in lower adherence rates than those of patients with gout.
Guidelines for the management of asymptomatic hyperuricemia in patients with CKD are conflicting. In the United States and Europe, routine ULT is not advised for asymptomatic hyperuricemia in CKD, although the 2019 Japanese guidelines recommend treatment at UA ≥ 8.0 mg/dL in patients with CKD [70,88,89]. Many Japanese and Korean nephrologists follow this proactive strategy for CKD stages 3-5 [90].
Although hyperuricemia may adversely affect renal function, the existing evidence is insufficient to recommend routine treatment for all patients with CKD and elevated UA levels. Nevertheless, several studies have indicated that certain subgroups benefit from ULT.
One possible indication for initiating ULT is a radiologically confirmed crystal deposition in the kidneys or vasculature. For instance, MSU crystals in renal tissue or arterial walls have been identified using advanced ultrasound or dual-energy computed tomography scans, suggesting subclinical organ damage even in asymptomatic hyperuricemia [31,32]. Additionally, patients with UA ≥ 9 mg/dL face an increased risk of nephrolithiasis and vascular complications, warranting an individualized approach [91,92].
Second, asymptomatic patients with hyperuricemia who lack proteinuria may benefit from treatment under certain conditions. In the FEATHER study, no clear effect on preventing the decline in renal function was found in any asymptomatic cases; however, febuxostat helped slow eGFR loss in CKD stage 3 among those without proteinuria [93,94]. This outcome implies that the proteinuria status could be crucial when deciding on therapy.
Third, urate crystals in the urine sediment support the initiation of treatment. For example, chronic ileostomy acidosis or familial hyperuricosuria with urinary urate crystals shows clinical improvement after receiving allopurinol or sodium bicarbonate [95,96]. Animal models have revealed that severe inflammation, including M1 macrophage activation and interstitial fibrosis, occurs only with intrarenal crystal deposition; the administration of anti-inflammatory agents helps alleviate this damage [30].
Finally, a cross-sectional study of Japanese health insurance data showed that a substantial proportion of asymptomatic patients with hyperuricemia received ULT (e.g., febuxostat or allopurinol). However, fewer than half achieve the target serum UA (≤ 6.0 mg/dL), suggesting that decisions to treat asymptomatic hyperuricemia may not always translate into optimal control in clinical practice and that more refined strategies might be required in future [97]. When managing hyperuricemia in patients with CKD, internists should be mindful of the risks of gout and UA nephrolithiasis. They should thoroughly review the patient's medical history and carefully examine the patient for any suggestive clinical features. If gout is strongly suspected, a referral for arthrocentesis is recommended. Additionally, renal ultrasonography can be considered a screening tool to detect UA stones. Comprehensive preventive measures, including lifestyle modifications, should be implemented. Furthermore, emerging research indicates that personalized treatment approaches, considering individual patient characteristics and comorbidities, could enhance the effectiveness of urate-lowering therapy and improve the overall outcomes of patients with hyperuricemia.
Despite the absence of overt symptoms, asymptomatic hyperuricemia may worsen CKD via crystal-dependent and crystal-independent pathways. However, current evidence does not support universal ULT in all cases, and trial results remain mixed. Specific subgroups, such as patients with CKD and radiologic crystal deposition, but no proteinuria or urate crystals in the urine sediment, may benefit from individualized therapy. Large-scale long-term studies to clarify who gains the most and improve adherence and outcomes are crucial in the future.
Notes
REFERENCES
1. Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One 2016;11:e0158765.



3. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 2021;143:1157–1172.



4. Johnson RJ, Bakris GL, Borghi C, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis 2018;71:851–865.



5. Ahn JK. Epidemiology and treatment-related concerns of gout and hyperuricemia in Korean. J Rheum Dis 2023;30:88–98.



6. Johnson RJ, Lozada LGS, Lanaspa MA, Piani F, Borghi C. Uric acid and chronic kidney disease: still more to do. Kidney Int Rep 2022;8:229–239.



7. Doria A, Galecki AT, Spino C, et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med 2020;382:2493–2503.



8. Badve SV, Pascoe EM, Tiku A, et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med 2020;382:2504–2513.


9. Yanai H, Adachi H, Hakoshima M, Katsuyama H. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int J Mol Sci 2021;22:9221.



11. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum 2011;63:3136–3141.


12. Álvarez-Lario B, Macarrón-Vicente J. Uric acid and evolution. Rheumatology (Oxford) 2010;49:2010–2015.


13. Antón FM, Puig JG, Ramos T, González P, Ordás J. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism 1986;35:343–348.


14. Stein CS, de Carvalho JAM, Duarte MMMF, et al. High serum uric acid is associated with oxidation of nucleosides in patients with type 2 diabetes. Mutat Res 2018;811:27–30.


15. Allegrini S, Garcia-Gil M, Pesi R, Camici M, Tozzi MG. The good, the bad and the new about uric acid in cancer. Cancers (Basel) 2022;14:4959.



16. Euser SM, Hofman A, Westendorp RG, Breteler MM. Serum uric acid and cognitive function and dementia. Brain 2009;132:377–382.


17. Martillo MA, Nazzal L, Crittenden DB. The crystallization of monosodium urate. Curr Rheumatol Rep 2014;16:400.



18. Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64:1431–1446.



19. Kim S, Hwang BH, Lee KY, et al. High uric acid levels in acute myocardial infarction provide better long-term prognosis predictive power when combined with traditional risk factors. J Clin Med 2022;11:5531.



20. Yu W, Cheng JD. Uric acid and cardiovascular disease: an update from molecular mechanism to clinical perspective. Front Pharmacol 2020;11:582680.



21. Ren H, Qu H, Zhang Y, Gu Y, Zhao Y, Xu W, et al. Detection of monosodium urate depositions and atherosclerotic plaques in the cardiovascular system by dual-energy computed tomography. Heliyon 2024;10:e24548.



22. Klauser AS, Strobl S, Schwabl C, et al. Prevalence of monosodium urate (MSU) deposits in cadavers detected by dual-energy computed tomography (DECT). Diagnostics (Basel) 2022;12:1240.



23. Emmerson BT, Cross M, Osborne JM, Axelsen RA. Ultrastructural studies of the reaction of urate crystals with a cultured renal tubular cell line. Nephron 1991;59:403–408.


24. Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum 2005;52:2936–2946.


25. Gicquel T, Robert S, Loyer P, et al. IL-1β production is dependent on the activation of purinergic receptors and NLRP3 pathway in human macrophages. FASEB J 2015;29:4162–4173.


26. Rashidi M, Simpson DS, Hempel A, et al. The pyroptotic cell death effector gasdermin D is activated by gout-associated uric acid crystals but is dispensable for cell death and IL-1β release. J Immunol 2019;203:736–748.



27. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237–241.


28. So AK, Martinon F. Inflammation in gout: mechanisms and therapeutic targets. Nat Rev Rheumatol 2017;13:639–647.


29. Ayoub I, Almaani S, Brodsky S, et al. Revisiting medullary tophi: a link between uric acid and progressive chronic kidney disease? Clin Nephrol 2016;85:109–113.


30. Sellmayr M, Petzsche MRH, Ma Q, et al. Only hyperuricemia with crystalluria, but not asymptomatic hyperuricemia, drives progression of chronic kidney disease. J Am Soc Nephrol 2020;31:2773–2792.



31. Abou-Elela A. Epidemiology, pathophysiology, and management of uric acid urolithiasis: a narrative review. J Adv Res 2017;8:513–527.



32. Klauser AS, Halpern EJ, Strobl S, et al. Dual-energy computed tomography detection of cardiovascular monosodium urate deposits in patients with gout. JAMA Cardiol 2019;4:1019–1028.



33. Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol 2002;13:2888–2897.


34. Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001;38:1101–1106.


35. Stewart S, Dalbeth N, Vandal AC, Allen B, Miranda R, Rome K. Ultrasound features of the first metatarsophalangeal joint in gout and asymptomatic hyperuricemia: comparison with normouricemic individuals. Arthritis Care Res (Hoboken) 2017;69:875–883.


36. Borghi C, Rosei EA, Bardin T, et al. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens 2015;33:1729–1741; discussion 1741.


37. Khosla UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int 2005;67:1739–1742.


38. Kang DH, Nakagawa T. Uric acid and chronic renal disease: possible implication of hyperuricemia on progression of renal disease. Semin Nephrol 2005;25:43–49.


39. Oğuz N, Kırça M, Çetin A, Yeşilkaya A. Effect of uric acid on inflammatory COX-2 and ROS pathways in vascular smooth muscle cells. J Recept Signal Transduct Res 2017;37:500–505.


40. Ryu ES, Kim MJ, Shin HS, et al. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol 2013;304:F471–F480.


41. Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 2007;293:C584–C596.


42. Xiao J, Zhang XL, Fu C, et al. Soluble uric acid increases NALP3 inflammasome and interleukin-1β expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int J Mol Med 2015;35:1347–1354.


43. Abderrazak A, Syrovets T, Couchie D, et al. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol 2015;4:296–307.



44. Han HJ, Lim MJ, Lee YJ, Lee JH, Yang IS, Taub M. Uric acid inhibits renal proximal tubule cell proliferation via at least two signaling pathways involving PKC, MAPK, cPLA2, and NF-kappaB. Am J Physiol Renal Physiol 2007;292:F373–F381.

45. Yin W, Zhou QL, OuYang SX, Chen Y, Gong YT, Liang YM. Uric acid regulates NLRP3/IL-1β signaling pathway and further induces vascular endothelial cells injury in early CKD through ROS activation and K+ efflux. BMC Nephrol 2019;20:319.



46. Braga TT, Forni MF, Correa-Costa M, et al. Soluble uric acid activates the NLRP3 inflammasome. Sci Rep 2017;7:39884.



47. Zhao ZH, Xin FZ, Xue Y, et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp Mol Med 2019;51:1–14.



48. Kimura Y, Yanagida T, Onda A, Tsukui D, Hosoyamada M, Kono H. Soluble uric acid promotes atherosclerosis via AMPK (AMP-activated protein kinase)-mediated inflammation. Arterioscler Thromb Vasc Biol 2020;40:570–582.


49. Zhu P, Liu Y, Han L, Xu G, Ran JM. Serum uric acid is associated with incident chronic kidney disease in middle-aged populations: a meta-analysis of 15 cohort studies. PLoS One 2014;9:e100801.



50. Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: a systematic review and meta-analysis based on observational cohort studies. BMC Nephrol 2014;15:122.



51. Gonçalves DLN, Moreira TR, da Silva LS. A systematic review and meta-analysis of the association between uric acid levels and chronic kidney disease. Sci Rep 2022;12:6251.


52. Kuwabara M, Bjornstad P, Hisatome I, et al. Elevated serum uric acid level predicts rapid decline in kidney function. Am J Nephrol 2017;45:330–337.



53. Johnson RJ, Titte S, Cade JR, Rideout BA, Oliver WJ. Uric acid, evolution and primitive cultures. Semin Nephrol 2005;25:3–8.


54. Rashid I, Katravath P, Tiwari P, D’Cruz S, Jaswal S, Sahu G. Hyperuricemia - a serious complication among patients with chronic kidney disease: a systematic review and meta-analysis. Explor Med 2022;3:249–259.

55. Barman Z, Hasan M, Miah R, et al. Association between hyperuricemia and chronic kidney disease: a cross-sectional study in Bangladeshi adults. BMC Endocr Disord 2023;23:45.



56. Chen JH, Tsai CC, Liu YH, et al. Sex difference in the associations among hyperuricemia with new-onset chronic kidney disease in a large Taiwanese population follow-up study. Nutrients 2022;14:3832.



57. Chang PY, Chang YW, Lin YF, Fan HC. Sex-specific association of uric acid and kidney function decline in Taiwan. J Pers Med 2021;11:415.



58. Li X, Meng X, Timofeeva M, et al. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ 2017;357:j2376.



59. Jordan DM, Choi HK, Verbanck M, et al. No causal effects of serum urate levels on the risk of chronic kidney disease: a Mendelian randomization study. PLoS Med 2019;16:e1002725.



60. Gill D, Cameron AC, Burgess S, et al. Urate, blood pressure, and cardiovascular disease: evidence from Mendelian randomization and meta-analysis of clinical trials. Hypertension 2021;77:383–392.



61. Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 2010;5:1388–1393.



62. Hosoya T, Ohno I, Nomura S, et al. Effects of topiroxostat on the serum urate levels and urinary albumin excretion in hyperuricemic stage 3 chronic kidney disease patients with or without gout. Clin Exp Nephrol 2014;18:876–884.



63. Sircar D, Chatterjee S, Waikhom R, et al. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis 2015;66:945–950.


64. Nata N, Ninwisut N, Inkong P, Supasyndh O, Satirapoj B. Effects of febuxostat on markers of endothelial dysfunction and renal progression in patients with chronic kidney disease. Sci Rep 2023;13:13494.



65. Chen Q, Wang Z, Zhou J, et al. Effect of urate-lowering therapy on cardiovascular and kidney outcomes: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2020;15:1576–1586.


66. Sapankaew T, Thadanipon K, Ruenroengbun N, et al. Efficacy and safety of urate-lowering agents in asymptomatic hyperuricemia: systematic review and network meta-analysis of randomized controlled trials. BMC Nephrol 2022;23:223.



67. Bignardi PR, Ido DH, Garcia FAL, Braga LM, Delfino VDA. Does uric acid-lowering treatment slow the progression of chronic kidney disease? A meta-analysis of randomized controlled trials. Nefrologia (Engl Ed) 2023;43:167–181.


68. Luo Y, Song Q, Li J, et al. Effects of uric acid-lowering therapy (ULT) on renal outcomes in CKD patients with asymptomatic hyperuricemia: a systematic review and meta-analysis. BMC Nephrol 2024;25:63.



69. Casanova AG, Morales AI, Vicente-Vicente L, López-Hernández FJ. Effect of uric acid reduction on chronic kidney disease. Systematic review and meta-analysis. Front Pharmacol 2024;15:1373258.



70. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken) 2020;72:744–760.


71. Koike R, Kawakami Y, Kondo R, et al. Effect of dietary counseling on patients with asymptomatic hyperuricemia. J Med Invest 2023;70:34–40.


72. Wang M, Jiang X, Wu W, Zhang D. A meta-analysis of alcohol consumption and the risk of gout. Clin Rheumatol 2013;32:1641–1648.


73. Neogi T, Chen C, Niu J, Chaisson C, Hunter DJ, Zhang Y. Alcohol quantity and type on risk of recurrent gout attacks: an internet-based case-crossover study. Am J Med 2014;127:311–318.



74. Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2008;59:109–116.


75. Nguyen UD, Zhang Y, Louie-Gao Q, et al. Obesity paradox in recurrent attacks of gout in observational studies: clarification and remedy. Arthritis Care Res (Hoboken) 2017;69:561–566.



76. Stamp LK, Farquhar H, Pisaniello HL, et al. Management of gout in chronic kidney disease: a G-CAN consensus statement on the research priorities. Nat Rev Rheumatol 2021;17:633–641.



77. White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018;378:1200–1210.


78. Mackenzie IS, Ford I, Nuki G, et al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet 2020;396:1745–1757.


79. Deng H, Zhang BL, Tong JD, Yang XH, Jin HM. Febuxostat use and risks of cardiovascular disease events, cardiac death, and all-cause mortality: metaanalysis of randomized controlled trials. J Rheumatol 2021;48:1082–1089.

80. Fiori E, De Fazio L, Pidone C, et al. Asymptomatic hyperuricemia: to treat or not a threat? A clinical and evidence-based approach to the management of hyperuricemia in the context of cardiovascular diseases. J Hypertens 2024;42:1665–1680.


81. Park EH, Choi ST, Song JS. Current state and prospects of gout treatment in Korea. Korean J Intern Med 2022;37:719–731.



82. Rezapour A, Alidoost S, Asgharzadeh A, et al. Cost-effectiveness of allopurinol versus febuxostat in the treatment of gout patients: a systematic review. Med J Islam Repub Iran 2020;34:41.



83. Stamp LK, Chapman PT. Allopurinol hypersensitivity: pathogenesis and prevention. Best Pract Res Clin Rheumatol 2020;34:101501.


84. Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol 2020;16:380–390.


85. Scheepers LEJM, van Onna M, Stehouwer CDA, Singh JA, Arts ICW, Boonen A. Medication adherence among patients with gout: a systematic review and meta-analysis. Semin Arthritis Rheum 2018;47:689–702.


86. Kim JW, Kwak SG, Park SH. Prescription pattern of urate-lowering therapy in Korean gout patients: data from the national health claims database. Korean J Intern Med 2018;33:228–229.



87. Halpern R, Mody RR, Fuldeore MJ, Patel PA, Mikuls TR. Impact of noncompliance with urate-lowering drug on serum urate and gout-related healthcare costs: administrative claims analysis. Curr Med Res Opin 2009;25:1711–1719.


88. Hisatome I, Ichida K, Mineo I, et al. Japanese Society of Gout and Uric & Nucleic Acids 2019 guidelines for management of hyperuricemia and gout, 3rd edition. Gout and Uric & Nucleic Acids 2020;44 Suppl:1–40.
89. Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017;76:29–42.


90. Cha RH, Kim SH, Bae EH, et al. Physicians' perceptions of asymptomatic hyperuricemia in patients with chronic kidney disease: a questionnaire survey. Kidney Res Clin Pract 2019;38:373–381.



91. Kim S, Chang Y, Yun KE, et al. Development of nephrolithiasis in asymptomatic hyperuricemia: a cohort study. Am J Kidney Dis 2017;70:173–181.


92. Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the normative aging study. Am J Med 1987;82:421–426.


93. Kimura K, Hosoya T, Uchida S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis 2018;72:798–810.

94. Kataoka H, Mochizuki T, Ohara M, et al. Urate-lowering therapy for CKD patients with asymptomatic hyperuricemia without proteinuria elucidated by attribute-based research in the FEATHER Study. Sci Rep 2022;12:3784.


95. Randall RE Jr. Urate nephropathy following chronic ileostomy acidosis. Am J Nephrol 2002;22:372–375.


96. Praga M, Alegre R, Hernández E, et al. Familial microscopic hematuria caused by hypercalciuria and hyperuricosuria. Am J Kidney Dis 2000;35:141–145.


97. Koto R, Nakajima A, Horiuchi H, Yamanaka H. Real-world treatment of gout and asymptomatic hyperuricemia: a cross-sectional study of Japanese health insurance claims data. Mod Rheumatol 2021;31:261–269.


98. Stack AG, Dronamraju N, Parkinson J, et al. Effect of intensive urate lowering with combined verinurad and febuxostat on albuminuria in patients with type 2 diabetes: a randomized trial. Am J Kidney Dis 2021;77:481–489.



99. Wada T, Hosoya T, Honda D, et al. Uric acid-lowering and renoprotective effects of topiroxostat, a selective xanthine oxidoreductase inhibitor, in patients with diabetic nephropathy and hyperuricemia: a randomized, double- blind, placebo-controlled, parallel-group study (UPWARD study). Clin Exp Nephrol 2018;22:860–870.


UA metabolism and risk factors for hyperuricemia. Excessive intake of purine-rich foods, alcohol, fructose, obesity/chronic kidney disease, and certain medications leads to the breakdown of AMP and GMP into hypoxanthine and xanthine, which are subsequently converted to UA by xanthine oxidase. Unlike rodents, humans lack uricase, an enzyme that converts UA to allantoin. UA is primarily excreted via the kidneys (approximately 70%) and intestines (approximately 30%). CKD, chronic kidney disease; AMP, adenosine monophosphate; RNA, ribonucleic acid; DNA, deoxyribonucleic acid; GMP, guanosine monophosphate; UA, uric acid.
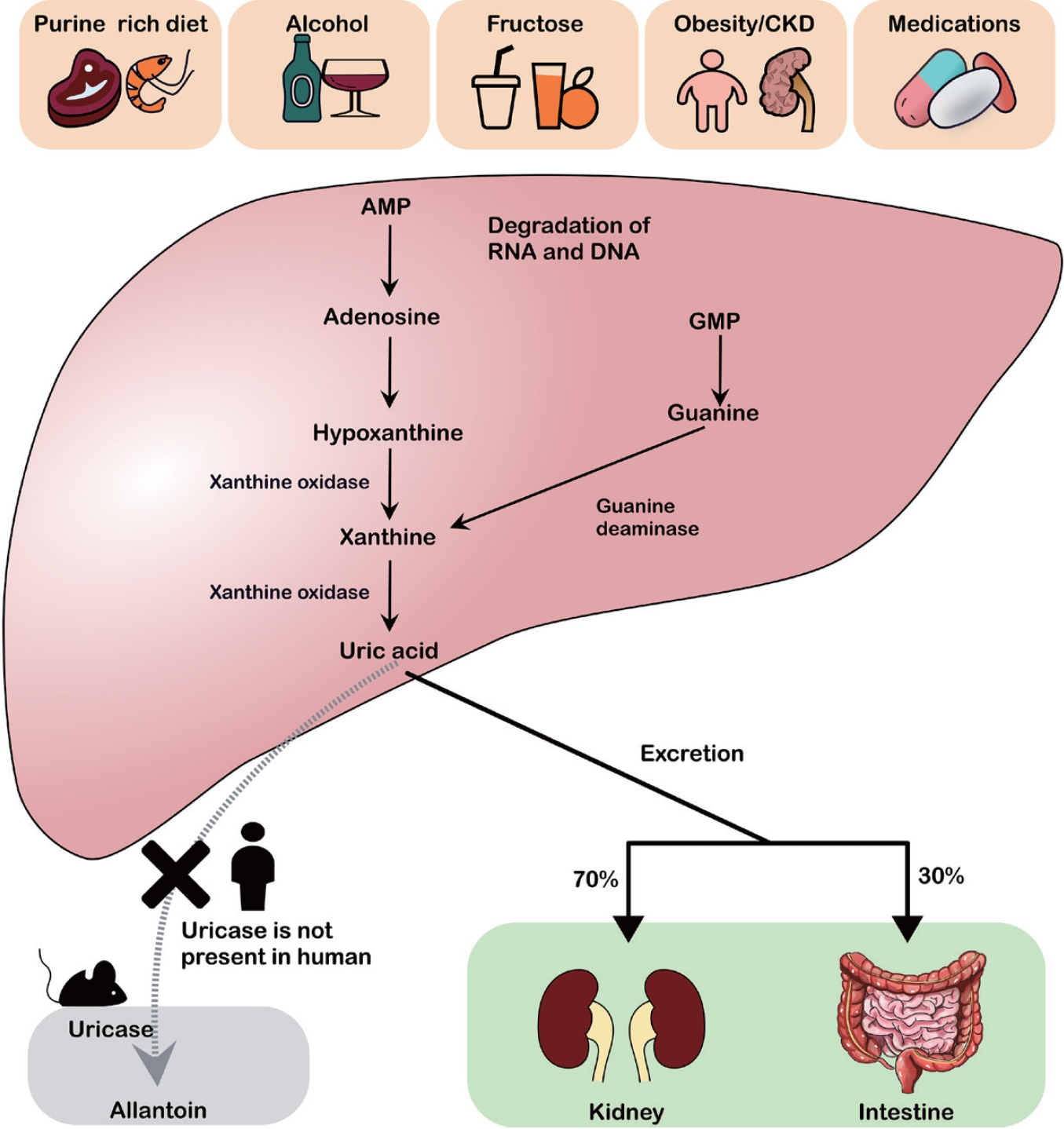
Figure 1.
Crystal-dependent and crystal-independent pathophysiology of hyperuricemia leading to CKD. In the crystal-dependent pathway, MSU crystals deposit in blood vessels, promoting atherosclerosis, or accumulate in renal structures, causing tubular obstruction and inflammation. In the crystal-independent pathway, soluble uric acid elevates oxidative stress, activates the RAAS, and reduces NO bioavailability, ultimately leading to glomerular hyperfiltration and endothelial dysfunction. Both pathways drive chronic inflammation and reduce renal perfusion, glomerular injury, and interstitial fibrosis, culminating in the development of CKD. MSU, monosodium urate; RAAS, renin-angiotensin-aldosterone system; NO, nitric oxide; CKD, chronic kidney disease.
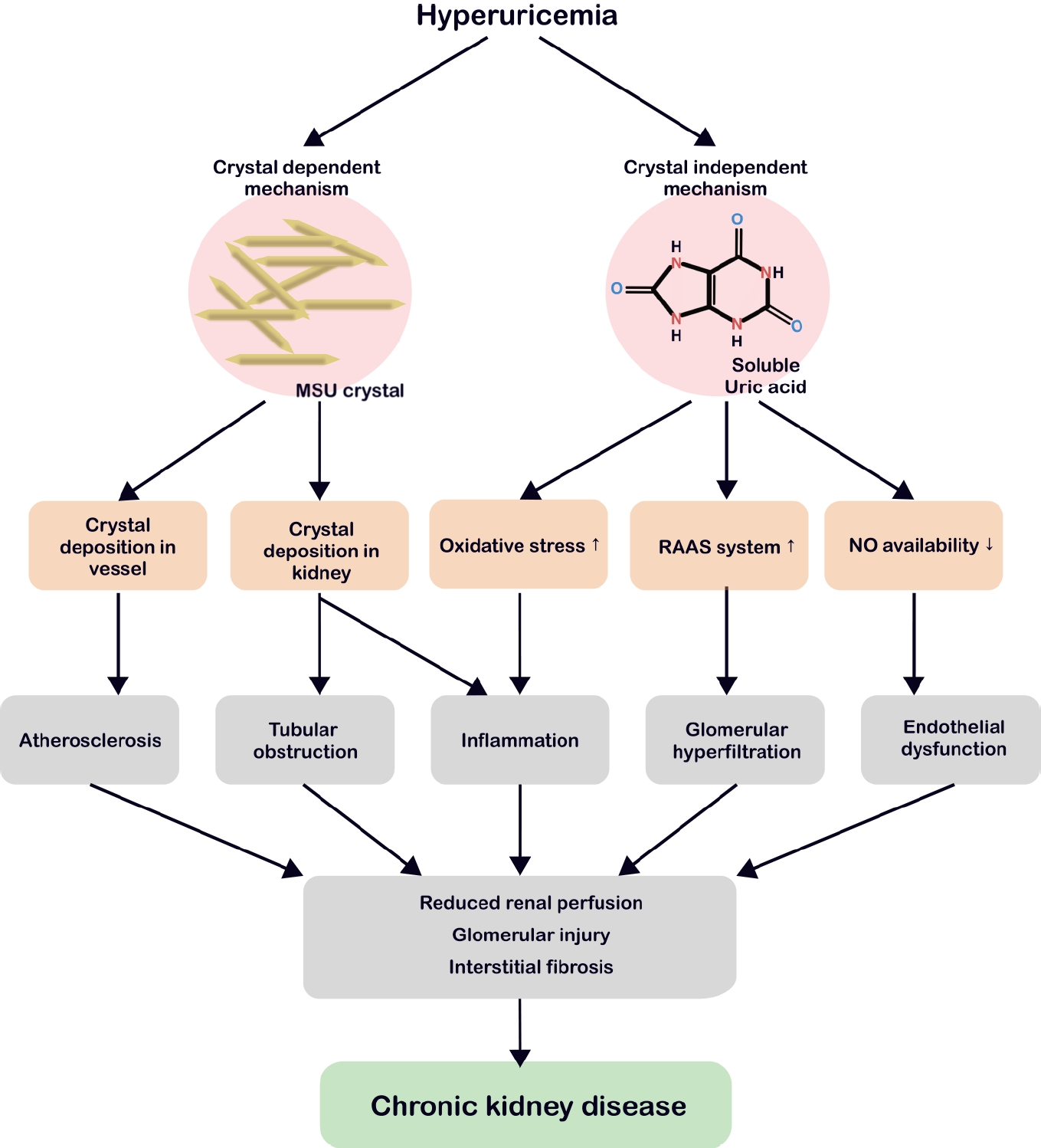
Figure 2.
MSU crystal-induced NLRP3 inflammasome activation in macrophages. MSU crystals activate the NLRP3 inflammasome via TLR2/4 and ROS production, leading to caspase-1 activation and release of mature IL-1β and IL-18. Activated caspase-1 triggers pyroptosis through GSDMD cleavage, which further amplifies inflammation. MSU, monosodium urate; TLR, toll-like receptor; MyD88, myeloid differentiation primary response 88; NADPH, nicotinamide adenine dinucleotide phosphate; ASC, apoptosis-associated speck-like protein containing a CARD; ROS, reactive oxygen species; NLRP3, nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3; NF-κB, nuclear factor kappa B; GSDMD, gasdermin D; IL, interleukin.

Figure 3.
Pathophysiological mechanisms linking hyperuricemia to chronic kidney disease progression. The schematic summarizes the harmful impact of hyperuricemia on the kidneys and vasculature, particularly within the proximal tubular epithelial cells, via RAAS and sympathetic system activation. Soluble uric acid drives endothelial dysfunction, systemic hypertension, and glomerular injury by suppressing NO bioavailability and activating inflammatory pathways (NF-κB, inflammasome). Intracellularly, uric acid impairs autophagy and mitochondrial function, exacerbating oxidative stress and renal fibrosis. HTN, hypertension; VSMC, vascular smooth muscle cell; RAAS, renin-angiotensin-aldosterone system; EMT, epithelial-mesenchymal transition; NO, nitric oxide; NADPH, nicotinamide adenine dinucleotide phosphate; URAT1, urate transporter 1; NOX4, NADPH oxidase 4; ROS, reactive oxygen species; AMPK, AMP-activated protein kinase; mTOR, mechanistic target of rapamycin; NF-κB, nuclear factor kappa B; TNF-α, tumor necrosis factor α; MCP1, monocyte chemoattractant protein-1; IL, interleukin.
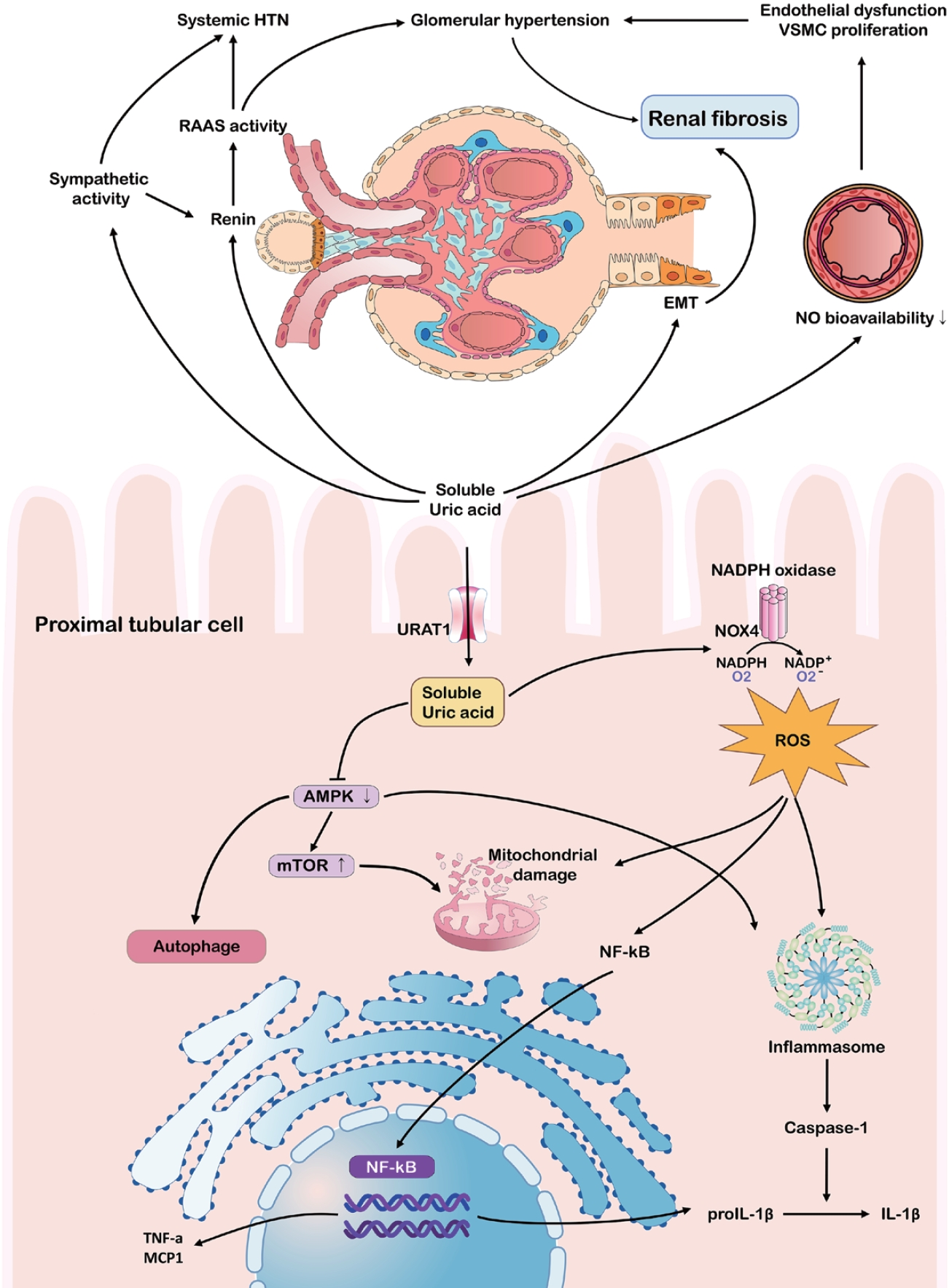
Figure 4.
Table 1.
Summary of clinical studies on uric acid-lowering therapies and their effects on kidney function
| Study | Number of patients (control/treatment) | Study population | Baseline SUA (mg/dL) | Final SUA (mg/dL) | Drug | FU (weeks) | Design | Location | Results |
|---|---|---|---|---|---|---|---|---|---|
| Nata et al. [64] (2023) | 84 (42/42) | CKD with hyperuricemia | 8.39 (control) | 8.03 (control) | Placebo/febuxostat 40 mg | 8 | RCT | Thailand | Significant SUA reduction (-3.4 mg/dL in treatment) but no significant eGFR improvement |
| 8.90 (treatment) | 5.49 (treatment) | ||||||||
| Stack et al. [98] (2021) | 60 (28/32) | CKD with hyperuricemia | 7.0 (placebo) | 7.3 (placebo) | Placebo/verinurad + febuxostat | 24 | Phase 2, multicenter, RCT | United States | Significant reduction in serum urate (-63.7%) and UACR (-49.3%) in the treatment group |
| 7.5 (treatment) | 4.1 (treatment) | ||||||||
| Doria et al. [7] (2020) | 530 (263/267) | Type 1 diabetes and CKD | 6.1 (both groups) | 6.1 (placebo) | Placebo/allopurinol (dose adjusted based on eGFR) | 156 | Multicenter, RCT | United States, Canada, Denmark | No significant effect of allopurinol on eGFR decline |
| 3.9 (treatment) | |||||||||
| Badve et al. [8] (2020) | 369 (184/185) | CKD without gout | 8.2 (both groups) | 8.2 (placebo) | Placebo/allopurinol (100-300 mg daily) | 104 | Multicenter, RCT | Australia, New Zealand | No significant effect of allopurinol on eGFR decline |
| 5.1 (treatment) | |||||||||
| Wada et al. [99] (2018) | 65 (22/43) | Diabetic nephropathy and hyperuricemia | 7.03 (placebo) | Not specified | Placebo/topiroxostat (40-160 mg/day) | 28 | RCT | Japan | Topiroxostat significantly reduced SUA and slowed eGFR decline |
| 7.25 (treatment) | |||||||||
| Sircar et al. [63] (2015) | 93 (48/45) | CKD and asymptomatic hyperuricemia | 8.2 (placebo) | 7.8 (placebo) | Placebo/febuxostat 40 mg | 24 | Single-center, RCT | India | Febuxostat slowed eGFR decline compared to that in placebo |
| 9.0 (treatment) | 5.2 (treatment) | ||||||||
| Hosoya et al. [62] (2014) | 122 (60/62) | CKD with hyperuricemia, with or without gout | 8.5 (both groups) | Not specified | Placebo/topiroxostat 160 mg | 22 | Multicenter, RCT | Japan | Topiroxostat significantly reduced SUA and urinary albumin excretion |
| Goicoechea et al. [61] (2010) | 113 (56/57) | CKD with cardiovascular risk factors | Not specified | Not specified | Placebo/allopurinol 100 mg daily | 104 | RCT | Spain | Allopurinol slowed CKD progression and reduced cardiovascular risk |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print