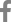|
 |
| Korean J Med > Volume 100(4); 2025 > Article |
|
мҡ”м•Ҫ
лӘ©м Ғ
м№ҙл°”нҺҳл„ҙ лӮҙм„ұ мһҘлӮҙм„ёк· мҶҚ(carbapenem-resistant Enterobacterales, CRE)м—җ мқҳн•ң к°җм—јмқҖ лӮ®мқҖ н•ӯк· м ң к°җмҲҳм„ұлҘ , м№ҳлЈҢм ңмқҳ л¶Җмһ‘мҡ©мңјлЎң мқён•ң лҶ’мқҖ мӮ¬л§қлҘ , м ңн•ңлҗң м№ҳлЈҢ мҳөм…ҳ л“ұмңјлЎң мқён•ҳм—¬ м „ м„ёкі„м ҒмңјлЎң мӨ‘лҢҖн•ң кіөмӨ‘ліҙкұҙ мң„нҳ‘мңјлЎң к°„мЈјлҗңлӢӨ. мөңк·ј лҸ„мһ…лҗң лІ нғҖлқҪнғҗ/лІ нғҖлқҪнғҖл§Ҳм ңм–өм ңм ңлі‘н•©м ң(ОІ-lactam/ОІ-lactamase inhibitors, BLBLIs)лҠ” нҠ№м • м№ҙл°”нҺҳл„ҙ분н•ҙнҡЁмҶҢ(carbapenemase) мғқм„ұ CREм—җ лҢҖн•ң 1м°Ё м№ҳлЈҢм ңлЎң нҷңмҡ©лҗҳкі мһҲлӢӨ. ліё м—°кө¬лҠ” көӯлӮҙ CRE к· мЈјлҘј лҢҖмғҒмңјлЎң BLBLIsм—җ лҢҖн•ң к°җмҲҳм„ұмқ„ нҸүк°Җн•ҳкі н•ӯк· м ң кҙҖлҰ¬ мөңм Ғнҷ” л°Ҹ лӮҙм„ұ к°җмӢңмқҳ мӨ‘мҡ”м„ұмқ„ к°•мЎ°н•ҳкі мһҗ н•ҳмҳҖлӢӨ.
л°©лІ•
Imipenem мөңмҶҢ м–өм ң лҶҚлҸ„ мёЎм • л°Ҹ imipenem, meropenem, ertapenemм—җ лҢҖн•ң л””мҠӨнҒ¬ нҷ•мӮ°лІ•мқ„ нҶөн•ҙ мҙқ 71 мЈјмқҳ CRE к· мЈјлҘј м„ лі„н•ҳмҳҖлӢӨ. лӘЁл“ CRE к· мЈјм—җ лҢҖн•ҙ ceftazidime-avibactam, imipenem-relebactam, meropenem-vaborbactamмқҳ к°җмҲҳм„ұ кІҖмӮ¬лҘј мӢңн–үн•ҳмҳҖкі лҸҷмӢңм—җ м№ҙл°”нҺҳл„ҙ분н•ҙнҡЁмҶҢ мң м „нҳ• 분м„қмқ„ мҲҳн–үн•ҳмҳҖлӢӨ.
кІ°кіј
мҙқ 71мЈјмқҳ CRE к· мЈј мӨ‘ KPC мғқм„ұ к· мЈјлҠ” 46мЈј, NDMнҳ•мқҖ 10мЈј, OXA-48-likeнҳ•мқҖ 2мЈјмҳҖмңјл©° лӮҳлЁём§Җ 13мЈјлҠ” м№ҙл°”нҺҳл„ҙ분н•ҙнҡЁмҶҢ 비мғқм„ұ(non-CP) CREлЎң нҷ•мқёлҗҳм—ҲлӢӨ. м „мІҙ CRE к· мЈјм—җ лҢҖн•ң ceftazidime-avibactam, imipenem-relebactam, meropenem-vaborbactamмқҳ к°җмҲҳм„ұлҘ мқҖ к°Ғк°Ғ 84.5%, 80.3%, 83.1%мҳҖлӢӨ. KPC мғқм„ұ к· мЈјм—җ лҢҖн•ҙм„ңлҠ” м„ё м•Ҫм ң к°Ғк°Ғ 97.8%, 93.5%, 95.7%мқҳ лҶ’мқҖ к°җмҲҳм„ұлҘ мқ„ ліҙмҳҖлӢӨ.
кІ°лЎ
мғҲлЎңмҡҙ BLBLIsлҠ” көӯлӮҙ CRE к· мЈјм—җ лҢҖн•ҙ м „л°ҳм ҒмңјлЎң лҶ’мқҖ к°җмҲҳм„ұлҘ мқ„ ліҙмҳҖмңјл©° нҠ№нһҲ KPC мғқм„ұ к· мЈјм—җм„ңлҠ” 90% мқҙмғҒмқҳ к°җмҲҳм„ұмқ„ ліҙмҳҖлӢӨ. мқҙлҹ¬н•ң кІ°кіјлҠ” CRE к°җм—ј м№ҳлЈҢм—җм„ң BLBLIsмқҳ мһ„мғҒм Ғ мң мҡ©м„ұмқ„ л’·л°ӣм№Ён•ҳл©° лӮҙм„ұ к· мЈјмқҳ мЎ°кё° л°ңкІ¬кіј нҷ•мӮ° л°©м§ҖлҘј мң„н•ң м§ҖмҶҚм Ғмқё к°җмӢң мІҙкі„ кө¬м¶•мқҙ н•„мҡ”н•Ёмқ„ мӢңмӮ¬н•ңлӢӨ.
Abstract
Background/Aims
Carbapenem-resistant Enterobacterales (CRE) infections represent a significant global health concern, characterized by low susceptibility rate- and adverse effect-related high mortality and limited treatment options. Novel beta-lactam/beta-lactamase inhibitors (BLBLIs) are first-line therapeutic agents against specific carbapenemase (CP)-producing CREs. We assessed current CRE susceptibility to these novel BLBLIs to aid optimal antimicrobial stewardship, emphasizing the need for continuous resistance surveillance.
Methods
In total, we identified 71 CRE isolates based on the imipenem minimum inhibitory concentration and disk diffusion susceptibility testing for imipenem, meropenem, and ertapenem. We performed antimicrobial susceptibility testing for novel BLBLIs, including ceftazidime-avibactam, imipenem-relebactam, and meropenem-vaborbactam as well as CP gene molecular characterization for all CRE isolates.
Results
We analysed a total of 71 CRE isolates and our CP genotypic analysis identified 46 Klebsiella pneumoniae CP (KPC)-producing, 10 New Delhi metallo-beta-lactamase-producing, and two oxacillinase-48-like producing isolates. We identified the remaining 13 isolates as non-CP-producing CREs. The susceptibility rates for ceftazidime-avibactam, imipenem-relebactam, and meropenem-vaborbactam were 84.5%, 80.3%, and 83.1%, respectively. Among the CP-producing isolates, KPC producers exhibited susceptibility rates of 97.8%, 93.5%, and 95.7% to ceftazidime-avibactam, imipenem-relebactam, and meropenem-vaborbactam, respectively.
Conclusions
Novel BLBLIs generally yielded high CRE susceptibility rates, with the latter exceeding 90% in KPC-producing isolates. Although these findings indicate the efficacy of novel BLBLIs in treating CRE infections, ongoing surveillance is essential to detect and mitigate resistant strain emergence.
Carbapenems, including imipenem, ertapenem, meropenem, and doripenem, were once considered the last resort for treating multidrug-resistant Gram-negative bacteria also resistant to broad-spectrum beta-lactams. However, following the development of imipenem in 1985, the first carbapenemase enzyme-producing Klebsiella pneumoniae was detected in 1996 and since then, carbapenem-resistant Enterobacterales (CRE) have been rapidly increasing worldwide. In Korea, since surveillance system expansion to nationwide monitoring in 2017, the number of CRE cases reported through the Korea Disease Control and Prevention AgencyвҖҷs Disease Health Management System has been continuously increasing. In 2024, a total of 42,347 cases (i.e., 2,747 patients and 39,600 carriers) were reported across the country [1]. CRE can be broadly classified into two resistance mechanism-based types: carbapenemase-producing Enterobacterales (CPE), which produce carbapenemase enzymes that directly degrade carbapenems, and non-carbapenemase-producing CRE, which acquire resistance through further mechanisms like the combination of AmpC ОІ-lactamase production and porin loss without producing carbapenemase. Approximately 71% of CRE cases documented in Korea reportedly belong to the CPE category, and 83% of the CRE cases were reported as CPE in the United States [2,3]. Carbapenemases are classified into four classes based on their amino acid sequences, including Ambler class A (K. pneumoniae carbapenemase, KPC), C, and D (oxacillinases, OXA), which require a serine residue for their activity, and Ambler class B (New Delhi metallo-ОІ-lactamases [NDM], imipenemase metallo-ОІ-lactamases [IMP], Verona integron-encoded metallo-ОІ-lactamase [VIM]), which requires zinc for its activity. While the CRE-produced carbapenemase types vary by region, approximately 77-80% of CPE cases in Korea and the United states reportedly produce KPC of ambler class A.
CRE treatment options remain significantly limited. Recently developed ОІ-lactam/ОІ-lactamase inhibitor combinations (BLBLIs) are highly effective against KPC-producing CRE. In Korea, the BLBLIs ceftazidime-avibactam has been introduced and is currently used, susceptibility testing for them is thus crucial for guiding treatment decisions and monitoring the emergence of new resistance. In this study, we aimed to investigate the susceptibility rates of CRE isolates from our institution to BLBLIs and other antimicrobial agents commonly used for CRE infection treatment.
From August 2024 to January 2025, the susceptibility rates of ОІ-lactam/ОІ-lactamase inhibitors and other antimicrobial agents were analyzed for CRE isolates collected from the laboratory. The strains were collected based on the minimum inhibitory concentration results obtained using the VITEKВ® 2 system (bioMГ©rieux, Marcy lвҖҷEtoile, France). CRE strains were defined as resistant to at least either imipenem or ertapenem according to the results of the antimicrobial susceptibility test conducted with the AST-N413 card (bioMГ©rieux). In addition, disc diffusion testing with imipenem, meropenem, and ertapenem was performed, and resistance was confirmed according to the Clinical and Laboratory Standards Institute (CLSI) guideline M100, ED34 [4] interpretative criteria. CRE strains were tested for susceptibility to BLBLIs and other antimicrobial agents using the AST-N439 card (bioMГ©rieux), and the susceptibility rates were analyzed based on the results. Furthermore, non-CP-CRE and CP-CRE were identified using the modified carbapenem inactivation method (mCIM). For all mCIM-positive isolates, carbapenemase subtype phenotypic testing was performed using the NG-TestВ® CARBA5 (NG Biotech, Guipry-Messac, France), which can detect and differentiate five major carbapenemase subtypes: KPC, NDM, IMP, VIM, and OXA-48-like enzymes. In addition, for isolates submitted to the Korea Health Environmental Institute for surveillance purposes, CPE result consistency from the institute was confirmed. In the Korea Health Environmental Institute, carbapenemase genes (KPC, NDM, OXA, IMP, VIM, Guiana extended spectrum ОІ-lactamase [GES], Sang Paulo metallo-ОІ-lactamase [SPM], German imipenemase [GIM], Seoul imipenemase [SIM], and Serratia marcescens enzymes [SME]) were identified using qualitative polymerase chain reaction, and, when necessary, specific genotypes were determined using sequencing. Supplementary sequencing- based molecular characterization was not carried out in our institution. The susceptibility rates for imipenem-relebactam, ceftazidime-avibactam, meropenem-vaborbactam, tigecycline, and fosfomycin were analyzed according to the carbapenemase enzyme phenotype. This study was approved by the Institutional Review Board (HPIRB NON2024-001-001).
In total, 71 CRE isolates were analyzed, including 54 K. pneumoniae, nine Escherichia coli , three Enterobacter spp., one Serratia marcescens, one Citrobacter freundii , one Citrobacter braakii , and one Enterobacter cloacae. Among these, carbapenemase (CP) gene typing revealed that 46 isolates were KPC producers, 10 isolates harbored NDM, and 2 isolates produced OXA-48-like. The remaining 13 isolates did not carry any CP genes and were classified as non-CP-producing CREs (Fig. 1).
The overall susceptibility rates of the 71 CRE isolates to ceftazidime- avibactam, imipenem-relebactam, and meropenem- vaborbactam were 84.5%, 80.3%, and 83.1%, respectively (Table 1). Among the CP-producing isolates, KPC-producing isolates demonstrated high susceptibility, with rates of 97.8%, 93.5%, and 95.7% to ceftazidime-avibactam, imipenem-relebactam, and meropenem-vaborbactam, respectively. All non-CP-producing CRE isolates exhibited complete susceptibility (100%) to both ceftazidime-avibactam and imipenem-relebactam. One non-CP-producing CRE isolate exhibited intermediate to meropenem-vaborbactam. Similarly, OXA-48-like-producing isolates also displayed 100% susceptibility to ceftazidime-avibactam and meropenem-vaborbactam. One OXA-48-like-producing isolate revealed imipenem-relebactam resistance. In contrast, NDM-producing isolates exhibited 100% resistance to ceftazidimeвҖ“avibactam, imipenem-relebactam, and one isolate exhibited susceptibility to meropenem-vaborbactam. Furthermore, CRE isolates retained susceptibility rates of 77.5% and 83.1% to tigecycline and fosfomycin, respectively. KPC-producing CRE susceptibility to tigecycline and fosfomycin was 82.6% and 84.8%, respectively.
In this study, we aimed to evaluate the susceptibility rates of newly developed and clinically used BLBLIs, including ceftazidime-avibactam. Among KPC-producing CRE, which represent the primary target pathogens for these agents, the susceptibility rates to ceftazidime-avibactam, imipenem-relebactam, and meropenem-vaborbactam were 97.8%, 93.5%, and 95.7%, respectively. A recent Korean study described similar results, with 184 out of 186 KPC-2-producing isolates (98.9%) susceptible to ceftazidime-avibactam, 173 (93.0%) to imipenem-relebactam, and 184 (98.9%) to meropenem-vaborbactam [5]. According to data reported from other countries, ceftazidime-avibactam demonstrated high in vitro activity, with 99.3% susceptibility against KPC-2-, OXA-48-like-, and imipenemase (IMI)-producing isolates, and 91.4% susceptibility among non-CP-CRE. As expected, no activity was observed against metallo-ОІ-lactamase (MBL)- or IMP-producing isolates [6]. Moreover, a domestic study first reported the acquired ceftazidime-avibactam resistance emergence during treatment in K. pneumoniae, underscoring the critical need for epidemiological monitoring and surveillance [7]. Similar resistance emergence trends upon ceftazidime-avibactam treatment have also been documented internationally [8]. Therefore, routine susceptibility testing for these new BLBLIs, still not widely implemented in a lot of clinical laboratories, should be prioritized to support appropriate antimicrobial stewardship.
Currently, various clinical studies are underway to develop new treatment options against CRE infections. For this purpose, certain antibiotics, although not yet introduced in Korea, have been approved and are currently in use in the United States and Europe. The CRE treatment strategy recommended by the Infectious Diseases Society of America (IDSA) involves using standard-spectrum antibiotics for which susceptibility has been confirmed through antimicrobial susceptibility testing. However, if resistance is identified against all standard-spectrum antibiotics, including carbapenems, treatment recommendations are based on CRE-produced carbapenemase enzyme presence and type [9]. In particular, for KPC-type carbapenemase, prevalent in Korea, the recommended treatment includes BLBLIs, such as ceftazidime-avibactam, meropenem-vaborbactam, and imipenem-relebactam. Alternative antibiotics include cefiderocol, tigecycline, and eravacycline (the latter two for intra-abdominal infections).
Unlike classic ОІ-lactamase inhibitors, acting as suicide substrates by sharing the ОІ-lactam ring structure, recently developed BLBLIs (e.g., avibactam, durlobactam, relebactam, and vaborbactam) inhibit ОІ-lactamases by forming covalent bonds with the serine residues in the active sites of the enzymes. Consequently, these inhibitors display their activity against serine beta-lactamases (e.g., KPC and OXA), whereas metallo-ОІ-lactamases (e.g., NDM, VIM, and IMP) are known to be resistant to these agents. In this study, KPC-producing isolates exhibited a very high susceptibility rate of 93.5-97.8% to all three BLBLIs, whereas NDM-producing isolates remained mostly resistant. Although OXA-type CREs were expected to be susceptible, the small number of isolates included in this study made drawing definitive conclusions difficult. Therefore, although KPC-producer resistance to BLBLIs observed in this study could be attributed to a major error in testing or mutation, further testing with the E-test revealed susceptibility, suggesting a potentially major error in the initial testing. In addition, although NDM-producing strains might be susceptible to meropenem-vaborbactam, these agents are presumably not active in vitro, thereby potentially necessitating BLBLIs with aztreonam.
Ceftazidime-avibactam is second-generation BLBLIs, which was approved in the United States in 2015. In Korea, it was launched as a non-reimbursed drug in July 2023 and has been included in the national health insurance reimbursement list since March 2024. Ceftazidime-avibactam can be used in cases when carbapenem antibiotics have failed, including complex intra-abdominal and urinary tract infections, hospital-acquired pneumonia, including ventilator-associated pneumonia, as well as in cases involving confirmed multidrug-resistant Pseudomonas aeruginosa or CRE. Therefore, ceftazidime-avibactam use is anticipated to increase in the future, and with this in mind, the importance of antimicrobial susceptibility testing has also come to the forefront. Although ceftazidime-avibactam application has not yet become widespread in Korea, KPC-type CREs have already been detected at very high levels. Therefore, data review of the appropriate ceftazidime-avibactam use and the corresponding resistance rates is required. According to an analysis by Wang et al., [10] Enterobacteriaceae demonstrate a notably low resistance to ceftazidime-avibactam with an overall resistance rate below 0.6%. P. aeruginosa exhibits lower susceptibility to ceftazidime-avibactam, with reported resistant rates ranging from 2.9% to 18.0%. In Acinetobacter baumannii , ceftazidime-avibactam resistance exceeds 50%. The European Centers for Disease Control and Prevention (CDC) has highlighted the risk of resistance to ceftazidime-avibactam in CRE, and capacity and method enhancement of clinical microbiology laboratories for ceftazidime-avibactam resistance detection as well as epidemiological surveillance improvement is recommended to mitigate this risk [11]. In addition, based on data from the 2023 national antimicrobial resistance surveillance report in Korea [12], the KPC-producing K. pneumoniae resistance rate to ceftazidime-avibactam was 5.5%. Therefore, monitoring the current ceftazidime-avibactam resistance rates in Korea as well as those of other newly developed BLBLIs is important.
While BLBLIs response can be estimated based on the isolated CPE types by different institutions, it is crucial to note that new BLBLIs exhibit resistance or susceptibility patterns specific to certain CRE types. Therefore, for the efficient use of these agents, conduct tests to identify the CP type is imperative. Furthermore, even though BLBLIs currently yield high susceptibility rates, continuous surveillance is necessary to monitor the emergence of new resistance mechanisms. To support this, clinical microbiology laboratories should display the capacity to perform BLBI susceptibility testing.
Notes
REFERENCES
1. Korea Disease Control and Prevention Agency. Infectious disease portal [Internet]. Cheongju (KR): Korea Disease Control and Prevention Agency, c2019 [cited 2025 Feb 24]. Available from: https://dportal.kdca.go.kr/pot/is/summaryEDW.do
2. Lim J, Sim J, Lee H, Hyun J, Lee S, Park S. Characteristics of carbapenem-resistant Enterobacteriaceae (CRE) in the Republic of Korea, 2022. Public Health Wkly Rep 2024;17:115вҖ“127.
3. Sader HS, Mendes RE, Carvalhaes CG, Kimbrough JH, Castanheira M. changing epidemiology of carbapenemases among carbapenem-resistant enterobacterales from United States hospitals and the activity of aztreonam-avibactam against contemporary Enterobacterales (2019-2021). Open Forum Infect Dis 2023;10:ofad046.



4. Lewis II JS, Mathers AJ, Bobenchik AM, et al. CLSI M100: performance standards for antimicrobial susceptibility testing. 34th ed. Malvern (PA): Clinical and Laboratory Standards Institute, 2024.
5. Kang MS, Baek JY, Ko JH, et al. Antimicrobial activity of ceftazidime-avibactam against KPC-2-producing Enterobacterales: a cross-combination and dose-escalation titration study with relebactam and vaborbactam. Microbiol Spectr 2024;12:e0034424.



6. Lim TP, Ho JY, Teo JQ, et al. In vitro susceptibility to ceftazidime-avibactam and comparator antimicrobial agents of carbapenem-Resistant Enterobacterales isolates. Microorganisms 2023;11:2158.



7. Won EJ, Park K, Jeong YS, et al. Evolution of blaKPC under the pressure of carbapenems and ceftazidime/avibactam in a patient with persistent bacteremia caused by Klebsiella pneumoniae. J Korean Med Sci 2024;39:e208.



8. Campogiani L, Vitale P, Lodi A, et al. Resistance to ceftazidime/avibactam in Klebsiella pneumoniae KPC-producing isolates: a real-life observational study. Antibiotics (Basel) 2023;12:820.



9. Tamma PD, Heil EL, Justo JA, Mathers AJ, Satlin MJ, Bonomo RA. Infectious Diseases Society of America 2024 guidance on the treatment of antimicrobial-resistant Gram-negative infections. Clin Infect Dis 2024 Aug 7 [Epub]. https://doi.org/10.1093/cid/ciae403


10. Wang Y, Wang J, Wang R, Cai Y. Resistance to ceftazidime-avibactam and underlying mechanisms. J Glob Antimicrob Resist 2020;22:18вҖ“27.


11. European Centre for Disease Prevention and Control. Emergence of resistance to ceftazidime-avibactam in carbapenem-resistant Enterobacteriaceae [Internet]. Solna (SE): European Centre for Disease Prevention and Control, c2018 [cited 2025 Feb 24]. Available from: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-emergence-resistance-ceftazidime-avibactam-carbapenem
12. Korean Disease Control and Prevention Agency. National antimicrobial resistance surveillance in Korea: 2023 annual report [Internet]. Cheongju (KR): Korea Disease Control and Prevention Agency, c2024 [cited 2025 Feb 24]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20310030000&bid=0132&act=view&list_no=726816&tag=&nPage=1
The types of strains included in this study and carbapenemase gene distributions. KPC, Klebsiella pneumoniae carbapenemase; OXA, oxacillinase; NDM, New Delhi metallo-ОІ-lactamases.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print