만성 콩팥병에서 고요산혈증은 왜 중요한가?
Hyperuricemia: Does it Matter in Chronic Kidney Disease?
Article information
Trans Abstract
Chronic kidney disease (CKD) affects approximately 10-15% of adults globally and is a significant public health issue owing to its association with cardiovascular disease, end-stage kidney disease, and high healthcare costs. Hyperuricemia has emerged as an important modifiable risk factor influencing CKD progression. Elevated uric acid (UA) levels contribute to kidney injury through crystal-dependent mechanisms, including monosodium urate crystal deposition and NLRP3 inflammasome activation, and crystal-independent pathways, such as endothelial dysfunction, activation of the renin-angiotensin-aldosterone system, and oxidative stress. Observational studies have consistently linked hyperuricemia to an increased risk of CKD onset and accelerated disease progression. Nevertheless, randomized controlled trials and meta-analyses investigating UA-lowering therapy (ULT) for asymptomatic hyperuricemia have yielded conflicting results regarding its effectiveness in slowing CKD progression. Clinical guidelines also differ: Japanese guidelines recommend ULT for serum UA levels exceeding 8.0 mg/dL, whereas Western guidelines generally do not support routine treatment of asymptomatic hyperuricemia. Thus, there remains a clear need for large-scale, long-term studies to define patient subgroups most likely to benefit from ULT and guide individualized treatment approaches.
INTRODUCTION
Chronic kidney disease (CKD) affects approximately 10-15% of adults worldwide and imposes a major public health burden because of its association with cardiovascular events, end-stage kidney disease (ESKD), and elevated healthcare costs [1-3]. To address this burden, interventions aimed at factors that trigger or worsen CKD would be required; hyperuricemia is one such factor [4].
Although high purine intake and alcohol consumption commonly lead to hyperuricemia, its prevalence has increased due to increased fructose consumption, obesity, metabolic syndrome, and the use of certain medications [5]. Although hyperuricemia is a key risk factor for gout, many patients remain asymptomatic. Reduced uric acid (UA) excretion can elevate serum levels in patients with CKD, potentially causing chronic inflammation and tissue injury via monosodium urate (MSU) crystal deposition [6].
Observational research suggests that hyperuricemia can independently increase the likelihood of CKD onset and progression. However, large-scale randomized controlled trials (RCTs) investigating asymptomatic hyperuricemia have yielded mixed results regarding whether lowering UA levels can effectively slow the course of CKD [7,8]. Such inconsistencies have prompted a debate on whether hyperuricemia serves as a direct pathogenic factor or merely reflects broader metabolic disturbances.
The current review aimed to summarize the key pathophysiological mechanisms linking hyperuricemia to CKD, highlighting recent clinical evidence and providing an evidence-based framework for managing asymptomatic hyperuricemia in routine practice.
URIC ACID METABOLISM AND HYPERURICEMIA
UA, the final product of purine metabolism, is predominantly synthesized in the liver. Approximately 70% of UA is excreted by the kidneys, whereas the remaining 30% is excreted through the intestines [9]. Purines originate from endogenous molecules (RNA, DNA, and ATP) or dietary sources (e.g., meat and fructose). In recent years, the increased intake of purine-rich foods, alcohol, and fructose combined with obesity, CKD, and certain medications has contributed to a steady increase in hyperuricemia [10,11].
Purine catabolism proceeds via two main pathways, adenine and guanine (Fig. 1). Adenosine monophosphate (AMP) and guanosine monophosphate are first converted to adenosine and guanosine by nucleotidase enzymes, and then further degraded to xanthine, which is subsequently converted to UA by xanthine oxidase. Most mammals (except humans and higher primates) and amphibians possess uricases to oxidize UA into allantoin, thereby avoiding a significant elevation of UA in the serum. Humans lack functional uricases, making them more susceptible to hyperuricemia [12].
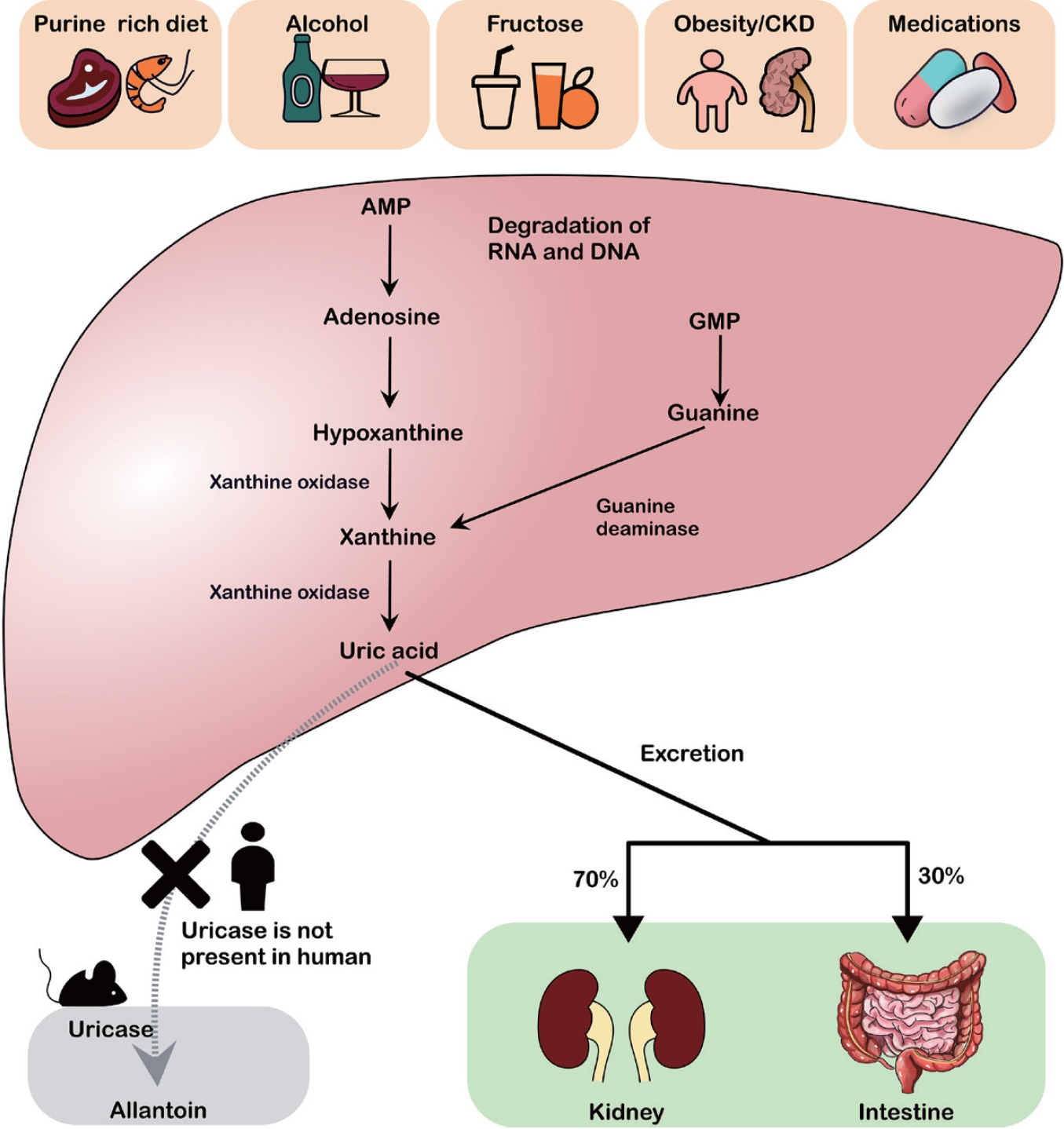
UA metabolism and risk factors for hyperuricemia. Excessive intake of purine-rich foods, alcohol, fructose, obesity/chronic kidney disease, and certain medications leads to the breakdown of AMP and GMP into hypoxanthine and xanthine, which are subsequently converted to UA by xanthine oxidase. Unlike rodents, humans lack uricase, an enzyme that converts UA to allantoin. UA is primarily excreted via the kidneys (approximately 70%) and intestines (approximately 30%). CKD, chronic kidney disease; AMP, adenosine monophosphate; RNA, ribonucleic acid; DNA, deoxyribonucleic acid; GMP, guanosine monophosphate; UA, uric acid.
There are notable sex-related differences in the UA metabolism, with men generally exhibiting higher serum UA levels than women. These differences can be attributed to various factors, including body composition. Men tend to have a larger body size and greater muscle mass, leading to increased purine turnover and UA production. Hormonal influences also contribute to the uricosuric effects of estrogen in women [13].
UA is a significant antioxidant, accounting for approximately half of the total antioxidant capacity of blood [14]. It may also play a role in the regulation of blood pressure under low-sodium conditions. Additional evidence suggests that it might as well protect against cancer and neurodegenerative diseases [15,16].
Serum UA concentrations roughly above 6.8 mg/dL is its solubility threshold, raising the risk of MSU crystal formation [17]. Hyperuricemia, often defined as > 7.0 mg/dL in men or 6.0 mg/dL in women [18], is strongly linked to gout besides being associated with cardiovascular disease, kidney disease, and metabolic syndrome [4,19]. Asymptomatic hyperuricemia without overt gout or nephrolithiasis is associated with a higher risk of cardiovascular disorders and CKD [20].
PATHOPHYSIOLOGY
Hyperuricemia-related renal injury involves both crystal-dependent and crystal-independent mechanisms (Fig. 2).
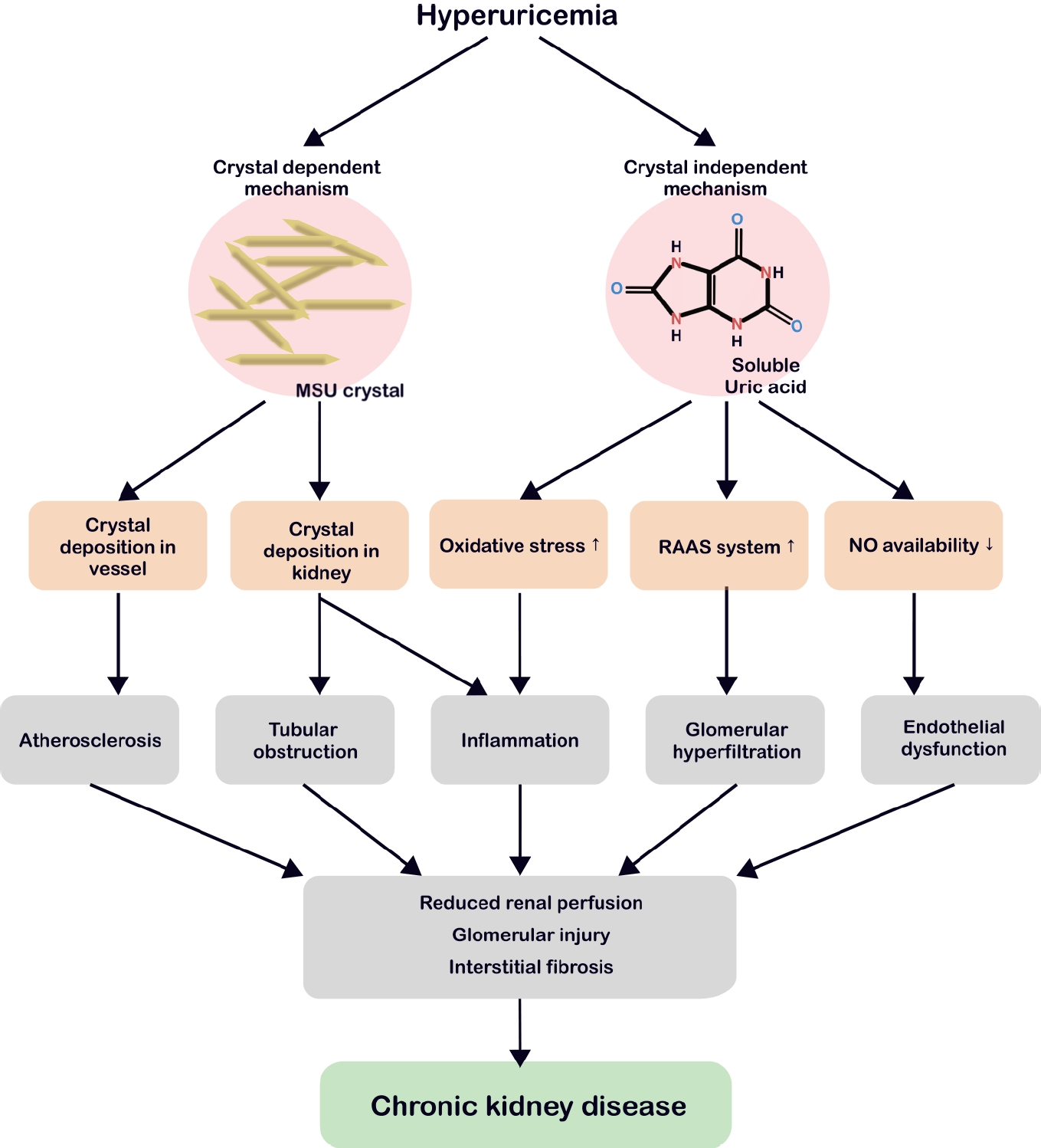
Crystal-dependent and crystal-independent pathophysiology of hyperuricemia leading to CKD. In the crystal-dependent pathway, MSU crystals deposit in blood vessels, promoting atherosclerosis, or accumulate in renal structures, causing tubular obstruction and inflammation. In the crystal-independent pathway, soluble uric acid elevates oxidative stress, activates the RAAS, and reduces NO bioavailability, ultimately leading to glomerular hyperfiltration and endothelial dysfunction. Both pathways drive chronic inflammation and reduce renal perfusion, glomerular injury, and interstitial fibrosis, culminating in the development of CKD. MSU, monosodium urate; RAAS, renin-angiotensin-aldosterone system; NO, nitric oxide; CKD, chronic kidney disease.
Crystal-dependent injury
Persistently elevated serum UA levels can result in the formation of MSU crystals that are deposited in the joints, blood vessels, and renal tissue [21-23]. MSU crystals bind toll-like receptor 2/4 on macrophages, triggering myeloid differentiation primary response 88 nuclear factor kappa B (NF-κB) signaling and inducing NOD-like receptor (NLR) family pyrin domain containing 3 (NLRP3) and pro-interleukin (IL)-1β expression (Fig. 3) [24]. Since macrophages engulf these crystals, heightened nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, cathepsin B leakage, and intracellular K+ depletion collectively activate the NLRP3 inflammasome [25]. Once active, the latter cleaves pro-IL-1β and pro-IL-18 into mature forms and splits gasdermin D, promoting pyroptosis and the release of inflammatory mediators [26].

MSU crystal-induced NLRP3 inflammasome activation in macrophages. MSU crystals activate the NLRP3 inflammasome via TLR2/4 and ROS production, leading to caspase-1 activation and release of mature IL-1β and IL-18. Activated caspase-1 triggers pyroptosis through GSDMD cleavage, which further amplifies inflammation. MSU, monosodium urate; TLR, toll-like receptor; MyD88, myeloid differentiation primary response 88; NADPH, nicotinamide adenine dinucleotide phosphate; ASC, apoptosis-associated speck-like protein containing a CARD; ROS, reactive oxygen species; NLRP3, nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3; NF-κB, nuclear factor kappa B; GSDMD, gasdermin D; IL, interleukin.
In the kidney, MSU crystal deposition triggers the overactivation of inflammasomes in the macrophages and neutrophils, elevating IL-1β and other cytokines [27,28]. Chronic urate nephropathy arises from granulomatous macrophage infiltration and persistent inflammation, culminating in tubular obstruction, interstitial fibrosis, and crystal cast formation at low pH [29-31]. Imaging studies have linked MSU crystals in blood vessels to accelerated atherosclerosis, suggesting similar risks of asymptomatic hyperuricemia [32].
Non-crystal-related injury
Some studies have indicated that the effects of hyperuricemia on CKD extend beyond crystal deposition [33,34]. Soluble UA can contribute to CKD progression via endothelial dysfunction, a heightened renin-angiotensin-aldosterone system (RAAS), and oxidative stress (Fig. 4). Hyperuricemia reduces nitric oxide synthase availability by inhibiting endothelial nitric oxide synthase, elevating vascular resistance, and promoting glomerular hypertension and tubulointerstitial damage [35]. Simultaneously, RAAS activation results in a hypertensive environment, while increased aldosterone and sodium reabsorption exacerbate glomerular strain [34,36-38].
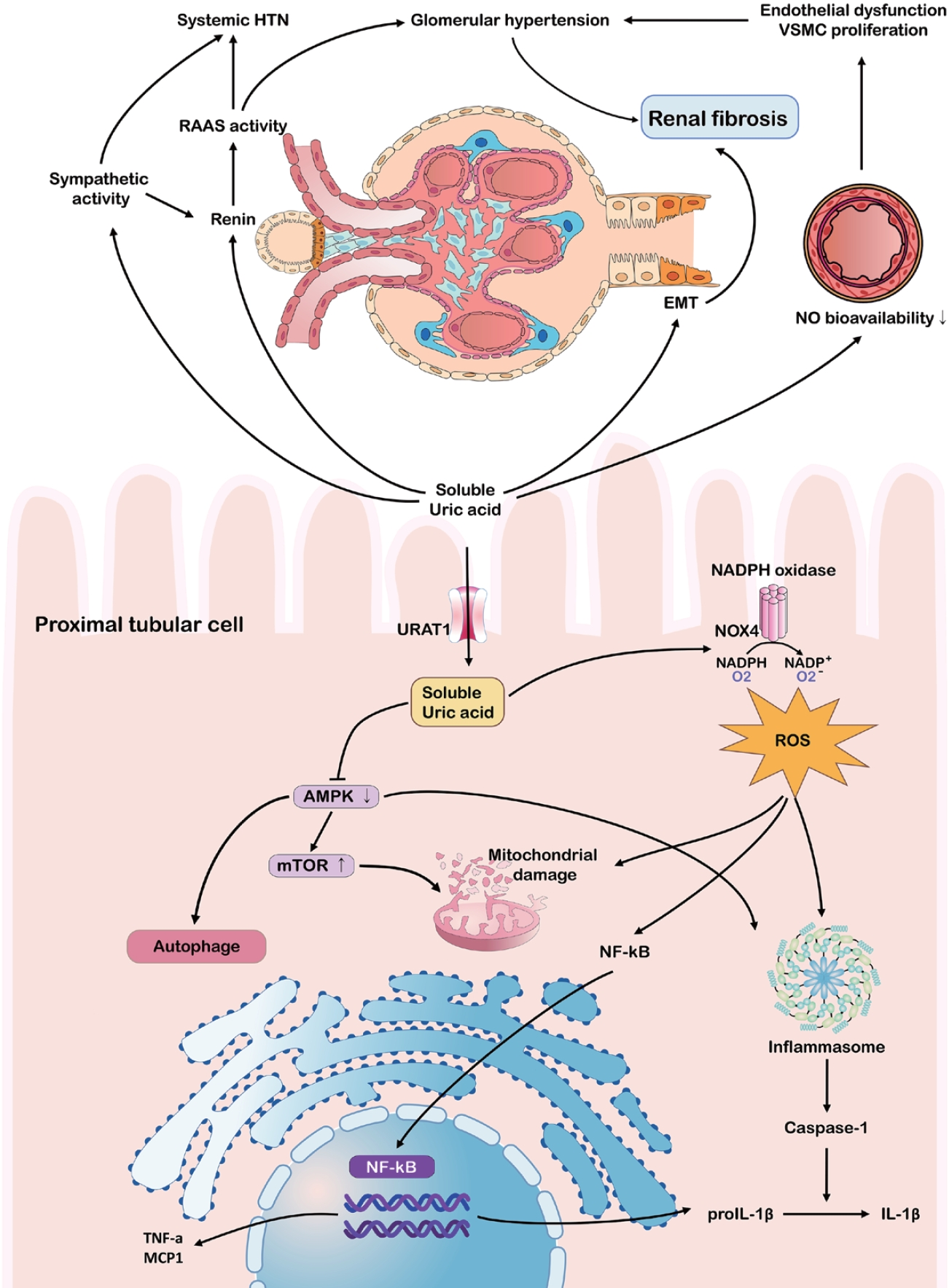
Pathophysiological mechanisms linking hyperuricemia to chronic kidney disease progression. The schematic summarizes the harmful impact of hyperuricemia on the kidneys and vasculature, particularly within the proximal tubular epithelial cells, via RAAS and sympathetic system activation. Soluble uric acid drives endothelial dysfunction, systemic hypertension, and glomerular injury by suppressing NO bioavailability and activating inflammatory pathways (NF-κB, inflammasome). Intracellularly, uric acid impairs autophagy and mitochondrial function, exacerbating oxidative stress and renal fibrosis. HTN, hypertension; VSMC, vascular smooth muscle cell; RAAS, renin-angiotensin-aldosterone system; EMT, epithelial-mesenchymal transition; NO, nitric oxide; NADPH, nicotinamide adenine dinucleotide phosphate; URAT1, urate transporter 1; NOX4, NADPH oxidase 4; ROS, reactive oxygen species; AMPK, AMP-activated protein kinase; mTOR, mechanistic target of rapamycin; NF-κB, nuclear factor kappa B; TNF-α, tumor necrosis factor α; MCP1, monocyte chemoattractant protein-1; IL, interleukin.
Soluble UA can further stimulate inflammatory pathways, including mitogen-activated protein kinases (MAPK) and cyclooxygenase-2, leading to the epithelial-mesenchymal transition in tubular cells, which is a key step in renal fibrosis [33,39,40]. Furthermore, UA elevates reactive oxygen species through NADPH oxidase, activating NLRP3 and NF-κB, and promoting chronic inflammation even without crystals [41-43]. This environment fosters mitochondrial damage and the release of cytokines like IL-1β and tumor necrosis factor-α, accelerating fibrosis [44-46]. Emerging data suggests that soluble UA inhibits AMP-activated protein kinase, drives mammalian target of rapamycin activation, and undermines autophagy, thereby exacerbating tubular cell damage [47,48].
EPIDEMIOLOGICAL TRENDS AND CLINICAL RELEVANCE OF HYPERURICEMIA IN CHRONIC KIDNEY DISEASE
Hyperuricemia as a risk factor for CKD development and progression
Several epidemiological studies and meta-analyses have indicated that serum UA levels strongly influence the incidence and progression of CKD. In a meta-analysis of 15 cohort studies (n = 99,205), each 1 mg/dL rise in UA increased CKD risk by 22%, notably among those under 60 years of age [49]. Another analysis of 190,718 individuals concluded that hyperuricemia was an independent predictor of CKD onset, especially in those without preexisting kidney disease [50]. A recent pooled analysis of 24 cohort studies (n = 412,238) found that a lower UA correlated with a relative risk of 0.44 compared to the highest UA group, suggesting significant renal protection [51]. Similarly, multiple long-term cohorts showed that elevated UA levels independently predicted estimated glomerular filtration rate (eGFR) decline [52].
Hyperuricemia is more common in patients with advanced CKD than in the general population, reaching 40-80% in some studies [53]. A meta-analysis of 23 studies (212,740 individuals) reported an overall hyperuricemia prevalence of 43.6% in patients with CKD, with higher rates in men (67.4%) than in women (32.6%) [54]. Similarly, a study conducted in Bangladesh reported that 23.2% of men and 14.6% of women with CKD have hyperuricemia [55].
In contrast, a cohort study in Taiwan demonstrated that hyperuricemia was associated with a higher odds ratio for new-onset CKD in women (3.813) than in men (1.989), suggesting a greater risk in women [56]. Additionally, each 1 mg/dL increase in serum UA was linked to a higher hazard ratio for renal function deterioration in women than in men, indicating that rising UA levels may have a more pronounced impact on CKD progression in women [57].
Although reduced glomerular filtration primarily contributes to impaired UA clearance, factors such as metabolic syndrome, obesity, and hypertension can further exacerbate hyperuricemia [36]. Elevated UA levels have been linked to a faster eGFR decline and a higher likelihood of ESKD [33].
CLINICAL EVIDENCE AND CONTROVERSIES SURROUNDING URIC ACID-LOWERING THERAPY IN CHRONIC KIDNEY DISEASE
Although observational studies have consistently linked hyperuricemia to CKD progression, evidence from Mendelian randomization (MR) studies has generated controversy regarding the causality. MR studies utilizing genetic variants as proxies failed to conclusively demonstrate a causal relationship between serum UA levels and CKD incidence or eGFR decline [58,59]. Furthermore, MR evidence regarding cardiovascular and hypertensive outcomes contradicts findings from observational research, raising further debate over whether hyperuricemia directly contributes to kidney damage or merely serves as a marker of broader metabolic dysfunction [60].
These conflicting findings highlight the complexity of the decision to initiate UA-lowering therapy (ULT) in patients with CKD. Although many RCTs have confirmed that ULT reduces serum UA levels, its overall impact on CKD progression remains controversial (Table 1). Some studies have suggested that allopurinol can slow CKD progression and lower cardiovascular risk [61], whereas topiroxostat and febuxostat have been associated with decreased proteinuria or slower eGFR decline [62,63].
The PERL trial in patients with type 1 diabetes and the CKD-FIX trial in patients with stage 3-4 CKD revealed that allopurinol did not demonstrate a significant benefit over placebo in preventing CKD progression [7,8]. These studies included populations whose mean UA levels were inconsistent with remarkable hyperuricemia, raising questions regarding their generalizability to real-world patients with more severe hyperuricemia [8]. Both the PERL and CKD-FIX included a substantial number of patients with normal serum UA levels and used intention-to-treat analyses, including those who discontinued treatment because of adverse events or non-adherence. Notably, approximately 17.5% to 30.0% of the participants dropped out before completing the study, which could have affected the final results. Nata et al. [64] also reported that febuxostat lowered serum UA levels but failed to remarkably improve eGFR.
Meta-analyses yielded mixed outcomes; one found no clear benefit regarding major events or renal failure [65], whereas others noted improved renal function with allopurinol or febuxostat [66]. Long-term studies appeared more favorable, reporting a slower eGFR decline [67,68], although questions regarding dose dependence and agent selection persisted [69].
Overall, the varied outcomes suggest that CKD risk level, treatment duration, and ULT choice all modulate potential gains. Large-scale, long-term RCTs that stratify patients by renal function and additional risk factors are required to identify patients who benefit the most.
TREATMENT OF ASYMPTOMATIC HYPERURICEMIA IN CHRONIC KIDNEY DISEASE
Lifestyle and dietary modifications
Lifestyle modifications play a crucial role in the management of hyperuricemia. The American College of Rheumatology recommends limiting the intake of alcohol, purine-rich foods, and high-fructose corn syrup while encouraging weight loss [70]. A study conducted in Japan revealed that adherence to dietary and lifestyle recommendations before starting medication led to a 7.8% reduction in serum UA (SU) levels over a 6-month period [71].
Alcohol consumption increases the risk of gout in a dose-dependent manner, with beer and distilled spirits exerting particularly significant effects on SU levels [72,73]. Moreover, fructose intake, commonly through sweetened beverages and fruit juices, has been associated with an increased risk of hyperuricemia and gout flare-ups, highlighting the importance of limiting consumption [74].
Obesity is an important risk factor for gout. An increase in body mass index (BMI) > 5% may result in a 60% increase in the frequency of gout attacks, whereas a 5% decrease in BMI can lower this risk by approximately 40% [75]. Maintaining adequate hydration and, if necessary, using alkalinizing agents (e.g., sodium bicarbonate) can help prevent the formation of UA stones in acidic urine. However, in patients with CKD, lifestyle modifications alone may be insufficient to manage hyperuricemia effectively, as these patients often have impaired urate excretion [76].
UA-lowering therapy
The initiation of ULT in patients with CKD requires careful dose selection and monitoring. Xanthine oxidase inhibitors are a primary class of drugs, and allopurinol is commonly used because of its effectiveness and affordability. In patients with CKD, however, therapy starts at a low dose (e.g., 50-100 mg/day) and is titrated slowly while monitoring for hypersensitivity and renal function changes. Rare, but severe, allopurinol hypersensitivity syndrome may occur, especially in patients with renal impairment or specific ethnic backgrounds. Screening for HLA-B*58:01 is recommended for high-risk groups (e.g., Koreans and Chinese Han) [18].
Febuxostat, a selective xanthine oxidase inhibitor, lowers serum UA levels more effectively than allopurinol. Although the CARES study reported increased cardiovascular mortality in patients with gout and cardiovascular disease [77], subsequent trials (e.g., FAST study) demonstrated its non-inferiority to allopurinol [78,79]. In patients with advanced CKD, febuxostat may be preferred when the use of allopurinol and uricosuric agents is limited.
Probenecid, benzbromarone, and lesinurad increase renal UA excretion but have limited efficacy, low glomerular filtration rate, and potential toxicity [36]. Rasburicase and pegloticase are restricted to severe hyperuricemia or tumor lysis syndrome. Certain angiotensin II receptor blockers or sodium glucose cotransporter 2 inhibitors may also indirectly reduce UA [80].
Although the cost-effectiveness of ULT in patients with CKD remains controversial, studies primarily focusing on gout have consistently highlighted the efficacy and affordability of allopurinol. Nonetheless, in patients with CKD, initiating allopurinol treatment at a low dose (e.g., 50-100 mg/day) with close monitoring of hypersensitivity and renal function changes is critical. Considering that approximately 12% of Koreans carry the HLA-B58:01 allele, screening is recommended to reduce the risk of severe hypersensitivity reactions [81]. Furthermore, specific evaluations suggested that even after accounting for the expense of HLA-B*58:01 screening, febuxostat may be comparatively cost-effective in patients with CKD [82].
The potential for severe skin reactions and other complications, which may be more pronounced in patients with CKD, highlights the need for careful patient selection and vigilance when using allopurinol [83]. Ultimately, a personalized approach that considers each patient’s risk factors and the distinct characteristics of the available medications is crucial for choosing the most suitable urate-lowering therapy.
TREATMENT ADHERENCE ISSUES
Research on treatment adherence among individuals with asymptomatic hyperuricemia is sparse, and much of the available information is indirectly obtained from studies involving patients with gout. Generally, the adherence to ULT in the aforementioned patient population remains poor and varies considerably worldwide, ranging from 17% to 78% [84,85]. In Korea, data from the Health Insurance Review and Assessment Service between 2007 and 2015 showed that although over 80% of patients with gout received at least one ULT prescription, only 35% persisted with the medication for more than 5 years [86]. Similarly, large-scale retrospective analyses have found that more than half of patients with gout who initiated allopurinol therapy discontinued treatment, and adherence significantly impacted the achievement of target serum UA levels: only 22.5% to 27.8% of low-adherence patients reached the target (< 6 mg/dL), compared to 49.3% to 56.8% of high-adherence patients [87]. Moreover, the CARES study reported that approximately half of the patients with gout at elevated cardiovascular risk ceased trial medications, complicating efficacy assessments [77].
Factors contributing to low adherence in patients with gout include symptom improvement, concerns about side effects, and financial burdens. Given that patients with CKD and asymptomatic hyperuricemia do not experience acute symptoms, such as painful gout attacks, they may feel less compelled to continue treatment, potentially resulting in lower adherence rates than those of patients with gout.
CURRENT GUIDELINES AND RECOMMENDATIONS
Guidelines for the management of asymptomatic hyperuricemia in patients with CKD are conflicting. In the United States and Europe, routine ULT is not advised for asymptomatic hyperuricemia in CKD, although the 2019 Japanese guidelines recommend treatment at UA ≥ 8.0 mg/dL in patients with CKD [70,88,89]. Many Japanese and Korean nephrologists follow this proactive strategy for CKD stages 3-5 [90].
Although hyperuricemia may adversely affect renal function, the existing evidence is insufficient to recommend routine treatment for all patients with CKD and elevated UA levels. Nevertheless, several studies have indicated that certain subgroups benefit from ULT.
One possible indication for initiating ULT is a radiologically confirmed crystal deposition in the kidneys or vasculature. For instance, MSU crystals in renal tissue or arterial walls have been identified using advanced ultrasound or dual-energy computed tomography scans, suggesting subclinical organ damage even in asymptomatic hyperuricemia [31,32]. Additionally, patients with UA ≥ 9 mg/dL face an increased risk of nephrolithiasis and vascular complications, warranting an individualized approach [91,92].
Second, asymptomatic patients with hyperuricemia who lack proteinuria may benefit from treatment under certain conditions. In the FEATHER study, no clear effect on preventing the decline in renal function was found in any asymptomatic cases; however, febuxostat helped slow eGFR loss in CKD stage 3 among those without proteinuria [93,94]. This outcome implies that the proteinuria status could be crucial when deciding on therapy.
Third, urate crystals in the urine sediment support the initiation of treatment. For example, chronic ileostomy acidosis or familial hyperuricosuria with urinary urate crystals shows clinical improvement after receiving allopurinol or sodium bicarbonate [95,96]. Animal models have revealed that severe inflammation, including M1 macrophage activation and interstitial fibrosis, occurs only with intrarenal crystal deposition; the administration of anti-inflammatory agents helps alleviate this damage [30].
Finally, a cross-sectional study of Japanese health insurance data showed that a substantial proportion of asymptomatic patients with hyperuricemia received ULT (e.g., febuxostat or allopurinol). However, fewer than half achieve the target serum UA (≤ 6.0 mg/dL), suggesting that decisions to treat asymptomatic hyperuricemia may not always translate into optimal control in clinical practice and that more refined strategies might be required in future [97]. When managing hyperuricemia in patients with CKD, internists should be mindful of the risks of gout and UA nephrolithiasis. They should thoroughly review the patient's medical history and carefully examine the patient for any suggestive clinical features. If gout is strongly suspected, a referral for arthrocentesis is recommended. Additionally, renal ultrasonography can be considered a screening tool to detect UA stones. Comprehensive preventive measures, including lifestyle modifications, should be implemented. Furthermore, emerging research indicates that personalized treatment approaches, considering individual patient characteristics and comorbidities, could enhance the effectiveness of urate-lowering therapy and improve the overall outcomes of patients with hyperuricemia.
CONCLUSION
Despite the absence of overt symptoms, asymptomatic hyperuricemia may worsen CKD via crystal-dependent and crystal-independent pathways. However, current evidence does not support universal ULT in all cases, and trial results remain mixed. Specific subgroups, such as patients with CKD and radiologic crystal deposition, but no proteinuria or urate crystals in the urine sediment, may benefit from individualized therapy. Large-scale long-term studies to clarify who gains the most and improve adherence and outcomes are crucial in the future.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Su Hyun Kim solely conceived and designed the review, conducted the literature search, analyzed and interpreted the relevant studies, drafted the manuscript, revised it critically for important intellectual content, and approved the final version for publication.
ACKNOWLEDGEMENTS
None.
